Abstract
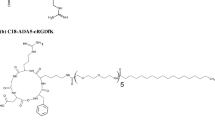
Decoration of nano-sized carriers with targeting ligands facilitates their cellular uptake in specific cells due to the ligand-receptor interaction and is being widely applied to fabricate nanoparticles for tumor-targeted therapy. In this chapter, we describe a strategy to covalently attach cyclo(Arg-Gly-Asp-D-Phe-Cys)(cRGD) peptide to a pH and redox potential dual-responsive micelle to realize tumor targeting. The micelle formation is based on the self-assembly of an amphiphilic polymer. The synthesis of the polymer and its post-modification including PEG-SH grafting and cRGD conjugation are comprehensively described. The fabrication of micelles and the investigation of its responsiveness to pH and redox potential are further introduced. Finally, the study of the targeting effect of cRGD micelles to αvβ5 integrin-overexpressed HCT 116 cells is also described.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Allen TM, Cullis PR (2013) Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev 65:36–48
Remant BK, Chandrashekaran V, Cheng B, Chen H, Peña MM, Zhang J, Montgomery J, Xu P (2014) Redox potential ultrasensitive nanoparticle for the targeted delivery of camptothecin to HER2-positive cancer cells. Mol Pharm 11:1897–1905
Bahadur KCR, Xu P (2012) Multicompartment intracellular self-expanding nanogel for targeted delivery of drug cocktail. Adv Mater 24:6479–6483
He H, Cattran AW, Nguyen T, Nieminen A-L, Xu P (2014) Triple-responsive expansile nanogel for tumor and mitochondria targeted photosensitizer delivery. Biomaterials 35:9546–9553
Bunjes H (2010) Lipid nanoparticles for the delivery of poorly water-soluble drugs. J Pharm Pharmacol 62:1637–1645
Moghimi SM, Hunter AC, Murray JC (2001) Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev 53:283–318
Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2:751–760
Sun Q, Sun X, Ma X, Zhou Z, Jin E, Zhang B, Shen Y, Van Kirk EA, Murdoch WJ, Lott JR, Lodge TP, Radosz M, Zhao Y (2014) Integration of nanoassembly functions for an effective delivery cascade for cancer drugs. Adv Mater 26:7615–7621
Motornov M, Roiter Y, Tokarev I, Minko S (2010) Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems. Prog Polym Sci 35:174–211
Cheng R, Meng F, Deng C, Klok H-A, Zhong Z (2013) Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 34:3647–3657
Zhuang J, Gordon MR, Ventura J, Li L, Thayumanavan S (2013) Multi-stimuli responsive macromolecules and their assemblies. Chem Soc Rev 42:7421–7435
Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC (2014) Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev 66:2–25
Zwicke GL, Mansoori GA, Jeffery CJ (2012) Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev 3:1–11
McGuire MJ, Gray BP, Li S, Cupka D, Byers LA, Wu L, Rezaie S, Liu Y-H, Pattisapu N, Issac J, Oyama T, Diao L, Heymach JV, Xie X-J, Minna JD, Brown KC (2014) Identification and characterization of a suite of tumor targeting peptides for non-small cell lung cancer. Sci Rep 4:1–11
Kocbek P, Obermajer N, Cegnar M, Kos J, Kristl J (2007) Targeting cancer cells using PLGA nanoparticles surface modified with monoclonal antibody. J Control Release 120:18–26
Jinushi M, Chiba S, Baghdadi M, Kinoshita I, Dosaka-Akita H, Ito K, Yoshiyama H, Yagita H, Uede T, Takaoka A (2012) ATM-mediated DNA damage signals mediate immune escape through integrin-αvβ3–dependent mechanisms. Cancer Res 72:56–65
Oba M, Fukushima S, Kanayama N, Aoyagi K, Nishiyama N, Koyama H, Kataoka K (2007) Cyclic RGD peptide-conjugated polyplex micelles as a targetable gene delivery system directed to cells possessing αvβ3 and αvβ5 integrins. Bioconjug Chem 18:1415–1423
Miura Y, Takenaka T, Toh K, Wu S, Nishihara H, Kano MR, Ino Y, Nomoto T, Matsumoto Y, Koyama H, Cabral H, Nishiyama N, Kataoka K (2013) Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood–brain tumor barrier. ACS Nano 7:8583–8592
Arosio D, Manzoni L, Araldi EMV, Scolastico C (2011) Cyclic RGD functionalized gold nanoparticles for tumor targeting. Bioconjug Chem 22:664–672
RB KC, Thapa B, Xu P (2012) pH and redox dual responsive nanoparticle for nuclear targeted drug delivery. Mol Pharm 9:2719–2729
Aied A, Zheng Y, Newland B, Wang W (2014) Beyond branching: multiknot structured polymer for gene delivery. Biomacromolecules 15:4520–4527
Dogan B, Catak S, Van Speybroeck V, Waroquier M, Aviyente V (2012) Free radical polymerization of ethyl methacrylate and ethyl α-hydroxy methacrylate: A computational approach to the propagation kinetics. Polymer 53:3211–3219
Wang Y, Zhou K, Huang G, Hensley C, Huang X, Ma X, Zhao T, Sumer BD, DeBerardinis RJ, Gao J (2014) A nanoparticle-based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat Mater 13:204–212
Chen D, Liu W, Shen Y, Mu H, Zhang Y, Liang R, Wang A, Sun K, Fu F (2011) Effects of a novel pH-sensitive liposome with cleavable esterase-catalyzed and pH-responsive double smart mPEG lipid derivative on ABC phenomenon. Int J Nanomedicine 6:2053–2061
Chuan X, Song Q, Lin J, Chen X, Zhang H, Dai W, He B, Wang X, Zhang Q (2014) Novel free-paclitaxel-loaded redox-responsive nanoparticles based on a disulfide-linked poly(ethylene glycol)–drug conjugate for intracellular drug delivery: synthesis, characterization, and antitumor activity in vitro and in vivo. Mol Pharm 11:3656–3670
Zheng Y, Ji S, Czerwinski A, Valenzuela F, Pennington M, Liu S (2014) FITC-conjugated cyclic RGD peptides as fluorescent probes for staining integrin αvβ3/αvβ5 in tumor tissues. Bioconjug Chem 25:1925–1941
Acknowledgments
This work is supported by the ASPIRE award from the Office of the Vice President for Research of the University of South Carolina, and the Center for Targeted Therapeutics (1P20GM109091-01) from National Institutes of Health (NIH).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this protocol
Cite this protocol
He, H., Bahadur K.C., R., Xu, P. (2015). Fabrication of cRGD-Conjugated Dual-Responsive Micelles to Target αvβ5 Integrin-Overexpressed Cancer. In: Patsenker, E. (eds) Integrin Targeting Systems for Tumor Diagnosis and Therapy. Methods in Pharmacology and Toxicology. Humana Press, New York, NY. https://doi.org/10.1007/7653_2015_42
Download citation
DOI: https://doi.org/10.1007/7653_2015_42
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-7443-6
Online ISBN: 978-1-4939-7445-0
eBook Packages: Springer Protocols