Abstract
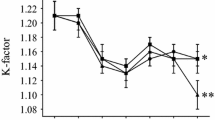
Previous experimental infection demonstrated that juvenile muskellunge (Esox masquinongy) can survive experimental infection of viral hemorrhagic septicemia virus, Genotype IVb (VHSV IVb) at a low concentration of exposure. Herein we report that survivors of experimental infection with VHSV IVb shed the virus into the surrounding environment for an extended period of time. When muskellunge were exposed to VHSV IVb by immersion at a concentration of 1,400 plaque forming units (PFU)/ml, VHSV IVb was detected in the water of surviving fish for up to 15 weeks postexposure (p.e.) with the highest levels of shedding occurring between weeks 1 and 5 p.e. We estimated that each juvenile muskellunge can shed upwards of 1.36×105 PFU/fish/h after initial exposure signifying the uptake and amplification of VHSV to several orders of magnitude above the original exposure concentration. Muskellunge surviving low concentration exposure were re-infected with VHSV IVb by immersion at week 22 p.e. at concentrations ranging from 0 to 106 PFU/ml. Viral shedding was detected in all re-exposed fish, including mock rechallenged controls up to 15 consecutive weeks. Rates of viral shedding were substantially higher following rechallenge in the first 5 weeks. The highest rate of viral shedding was approximately 4.6×106 PFU/fish/h and shedding did not necessarily correspond to the re-exposure VHSV concentration. The results of this study shed new light into the dynamics of VHSV IVb shedding in a highly susceptible host and provide useful insights to fishery managers to design effective control strategies to this deadly virus.
Similar content being viewed by others
References
Al-Hussinee, L., Huber, P., Russell, S., LePage, V., Reid, A., Young, K.M., Nagy, E., Stevenson, R.M.W., and Lumsden, J.S. 2010. Viral haemorrhagic septicaemia virus IVb experimental infection of rainbow trout, Oncorhynchus mykiss (Walbaum), and fathead minnow, Pimephales promelas (Rafinesque). J. Fish. Dis. 33, 347–360.
Batts, W.N., Traxler, G.S., and Winton, J.R. 1991. Factors affecting the efficiency of plating for selected fish rhabdoviruses, pp. 17–24. In Fryer, J.L. (ed.). Proceedings of the Second International Symposium on Viruses of Lower Vertebrates, Oregon State University Press, Corvallis, OR, USA.
Batts, W.N. and Winton, J.R. 1989. Enhanced detection of infectious hematopoietic necrosis virus and other fish viruses by pretreatment of cell monolayers with polyethylene glycol. J. Aquat. Anim. Health 1, 284–290.
Bowden, T.J. 2003. A study of the susceptibility of Atlantic halibut, Hippoglossus hippoglossus (L.), to viral haemorrhagic septicaemia virus isolated from turbot, Scophthalmus maximus (L.). J. Fish. Dis. 26, 207–212.
Brudeseth, B.E., Raynard, R.S., King, J.A., and Evensen, Ø. 2005. Sequential pathology after experimental infection with marine viral hemorrhagic septicemia virus isolates of low and high virulence in turbot (Scophthalmus maximus L.) Vet. Pathol. 42, 9–18.
de Kinkelin, P. and Castric, J. 1982. An experimental study of the susceptibility of Atlantic salmon fry, Salmo salar L., to viral haemorrhagic septicaemia. J. Fish. Dis. 5, 57–65.
Elsayed, E., Faisal, M., Thomas, M., Whelan, G., Batts, W., and Winton, J. 2006. Isolation of viral haemorrhagic septicaemia virus from muskellunge, Esox masquinongy (Mitchill), in Lake St. Clair, Michigan, USA reveals a new sublineage of the North American genotype. J. Fish. Dis. 29, 611–619.
Faisal, M. and Schulz, C.A. 2009. Detection of viral hemorrhagic septicemia virus (VHSV) from the leech Myzobdella lugubris Leidy, 1851. Parasit. Vectors 2, 45.
Faisal, M. and Winters, A.D. 2011. Detection of viral hemorrhagic septicemia virus (VHSV) from Diporeia spp. (Pontoporeiidae, Amphipoda) in the Laurentian Great Lakes, USA. Parasit. Vectors 4, 2.
Fijan, N., Sulimanovic, D., Bearzotti, M., Muzinic, D., Zwillenberg, L.O., Chilmonczyk, S., Vautherot, J.F., and de Kinkelin, P. 1983. Some properties of the epithelioma papulosum cyprini (EPC) cell line from carp (Cyprinus carpio). Ann. Inst. Pasteur Virol. 134, 207–220.
Hershberger, P., Gregg, J., Grady, C., Collins, R., and Winton, J. 2010. Kinetics of viral shedding provide insights into the epidemiology of viral hemorrhagic septicemia in Pacific herring. Mar. Ecol. Prog. Ser. 400, 187–193.
Hershberger, P.K., Gregg, J., Pacheco, C., Winton, J., Richard, J., and Traxler, G. 2007. Larval Pacific herring, Clupea pallasii (Valenceinnes) are highly susceptible to viral haemorrhagic septicaemia and survivors are partially protected after their metamorphosis to juvenile. J. Fish. Dis. 30, 445–458.
Kim, R.K. and Faisal, M. 2010a. The Laurentian Great Lakes strain (MI03) of the viral haemorrhagic septicaemia virus is highly pathogenic for juvenile muskellunge, Esox masquinongy (Mitchill). J. Fish. Dis. 33, 513–527.
Kim, R. and Faisal, M. 2010b. Experimental studies confirm the wide host range of the Great Lakes viral haemorrhagic septicaemia virus genotype IVb. J. Fish. Dis. 33, 83–88.
Kim, R. and Faisal, M. 2010c. Comparative susceptibility of representative Great Lakes fish species to the North American viral hemorrhagic septicemia virus Sublineage IVb. Dis. Aquat. Org. 91, 23–34.
Kocan, R., Bradley, M., Elder, N., Meyers, T., Batts, B., and Winton, J. 1997. North American strain of viral haemorrhagic septicaemia virus is highly pathogenic for laboratory-reared Pacific herring. J. Aquat. Anim. Health 9, 279–290.
López-Vázquez, C., Dopazo, C.P., Barja, J.L., and BandÍn, I. 2007. Experimental infection of turbot, Psetta maxima (L.), with strains of viral haemorrhagic septicaemia virus isolated from wild and farmed marine fish. J. Fish. Dis. 30, 303–312.
Muroga, K., Iida, H., Mori, K., Nishizawa, T., and Arimoto, M. 2004. Experimental horizontal transmission of viral hemorrhagic septicemia virus (VHSV) in Japanese flounder Paralichthys olivaceus. Dis. Aquat. Org. 58, 111–115.
Neukirch, M. and Glass, B. 1984. Some aspects of virus shedding by rainbow trout (Salmo gairdneri Rich.) after waterborne infection with viral haemorrhagic septicaemia (VHS) virus. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 257, 433–438.
Neukirch, M. 1985. Uptake, multiplication and excretion of viral hemorrhagic septicemia virus in trout (Salmo gairdneri), pp. 295–300. In Ellis, A.E. (ed.). Fish and Shellfish Pathology, Academic Press, London, UK.
Neukirch, M. 1986. Demonstration of persistent viral haemorrhagic septcaemia (VHS) virus in rainbow trout after experimental waterborne infection. Zentralbl. Veterinarmed. B 33, 471–476.
Reed, L.J. and Muench, H. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27, 493–497.
Skall, H.F., Slierendrecht, W.J., King, J.A., and Olesen, N.J. 2004. Experimental infection of rainbow trout Oncorhynchus mykiss with viral haemorrhagic septicaemia virus isolates from European marine and farmed fish. Dis. Aquat. Org. 58, 99–110.
Snow, M., Cunningham, C.O., and Bricknell, I.R. 2000. Susceptibility of juvenile Atlantic cod Gadus morhua to viral haemorrhagic septicaemia virus isolated from wild-caught Atlantic cod. Dis. Aquat. Org. 41, 225–229.
Wolf, K. 1988. Viral hemorrhagic septicemia, pp. 217–249. In Wolf, K. (ed.), Fish Viruses and Fish Viral Diseases, Comstock Publishing Associates, Cornell University Press, Ithaca, N.Y., USA.
World, Organization for Animal Health (OIE). 2003. In Vallat, B. (ed.), Chapter 2.1.5, Viral Haemorrhagic Septicaemia. Manual of Diagnostic Tests for Aquatic Animals. Office International des Epizooties, Paris, France.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, R.K., Faisal, M. Shedding of viral hemorrhagic septicemia virus (Genotype IVb) by experimentally infected muskellunge (Esox masquinongy). J Microbiol. 50, 278–284 (2012). https://doi.org/10.1007/s12275-012-1145-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-012-1145-2