Abstract
Background
Sublobar resection is strongly associated with poor prognosis in early-stage lung adenocarcinoma, with the presence of tumor spread through air spaces (STAS). Thus, preoperative prediction of STAS is important for surgical planning. This study aimed to develop a STAS deep-learning (STAS-DL) prediction model in lung adenocarcinoma with tumor smaller than 3 cm and a consolidation-to-tumor (C/T) ratio less than 0.5.
Methods
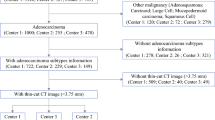
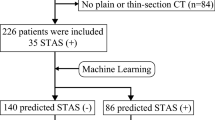
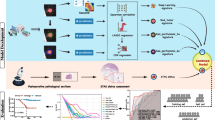
The study retrospectively enrolled of 581 patients from two institutions between 2015 and 2019. The STAS-DL model was developed to extract the feature of solid components through solid components gated (SCG) for predicting STAS. The STAS-DL model was assessed with external validation in the testing sets and compared with the deep-learning model without SCG (STAS-DLwoSCG), the radiomics-based model, the C/T ratio, and five thoracic surgeons. The performance of the models was evaluated using area under the curve (AUC), accuracy and standardized net benefit of the decision curve analysis.
Results
The study evaluated 458 patients (institute 1) in the training set and 123 patients (institute 2) in the testing set. The proposed STAS-DL yielded the best performance compared with the other methods in the testing set, with an AUC of 0.82 and an accuracy of 74%, outperformed the STAS-DLwoSCG with an accuracy of 70%, and was superior to the physicians with an AUC of 0.68. Moreover, STAS-DL achieved the highest standardized net benefit compared with the other methods.
Conclusion
The proposed STAS-DL model has great potential for the preoperative prediction of STAS and may support decision-making for surgical planning in early-stage, ground glass-predominant lung adenocarcinoma.






Similar content being viewed by others
References
de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382:503–13.
Suzuki K, Koike T, Asakawa T, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol. 2011;6:751–6.
Ginsberg RJ, Rubinstein LV, Group LCS. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann Thorac Surg. 1995;60:615–23.
Chiang X-H, Lu T-P, Hsieh M-S, et al. Thoracoscopic wedge resection versus segmentectomy for cT1N0 lung adenocarcinoma. Ann Surg Oncol. 2021;28:8398–411.
Aokage K, Suzuki K, Saji H, et al. Segmentectomy for ground glass-dominant lung cancer with a tumour diameter of 3 cm or less including ground-glass opacity (JCOG1211): a multicentre, single-arm, confirmatory, phase 3 trial. Lancet Respir Med. 2023;11:540–9.
Altorki N, Wang X, Kozono D, et al. Lobar or sublobar resection for peripheral stage IA non–small-cell lung cancer. N Engl J Med. 2023;388:489–98.
Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet. 2022;399:1607–17.
Eguchi T, Kameda K, Lu S, et al. Lobectomy is associated with better outcomes than sublobar resection in spread through air spaces (STAS)-positive T1 lung adenocarcinoma: a propensity score–matched analysis. J Thorac Oncol. 2019;14:87–98.
Kadota K, Kushida Y, Kagawa S, et al. Limited resection is associated with a higher risk of locoregional recurrence than lobectomy in stage I lung adenocarcinoma with tumor spread through air spaces. Am J Surg Pathol. 2019;43:1033–41.
Hu S-Y, Hsieh M-S, Hsu H-H, et al. Correlation of tumor spread through air spaces and clinicopathological characteristics in surgically resected lung adenocarcinomas. Lung Cancer. 2018;126:189–93.
Chen D, Mao Y, Wen J, et al. Tumor spread through air spaces in non-small cell lung cancer: a systematic review and meta-analysis. Ann Thorac Surg. 2019;108:945–54.
Kadota K, Nitadori J-C, Sima CS, et al. Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol. 2015;10:806–14.
Walts AE, Marchevsky AM. Current evidence does not warrant frozen section evaluation for the presence of tumor spread through alveolar spaces. Arch Pathol Lab Med. 2018;142:59–63.
de Margerie-Mellon C, Onken A, Heidinger BH, VanderLaan PA, Bankier AA. CT manifestations of tumor spread through airspaces in pulmonary adenocarcinomas presenting as subsolid nodules. J Thorac Imaging. 2018;33:402–8.
Kim SK, Kim TJ, Chung MJ, et al. Lung adenocarcinoma: CT features associated with spread through air spaces. Radiology. 2018;289:831–40.
Toyokawa G, Yamada Y, Tagawa T, et al. Computed tomography features of resected lung adenocarcinomas with spread through air spaces. J Thorac Cardiovasc Surg. 2018;156:1670–6.
Jiang C, Luo Y, Yuan J, et al. CT-based radiomics and machine learning to predict spread through air space in lung adenocarcinoma. Eur Radiol. 2020;30:4050–7.
Chen D, She Y, Wang T, et al. Radiomics-based prediction for tumour spread through air spaces in stage I lung adenocarcinoma using machine learning. Eur J Cardiothorac Surg. 2020;58:51–8.
Zhuo Y, Feng M, Yang S, et al. Radiomics nomograms of tumors and peritumoral regions for the preoperative prediction of spread through air spaces in lung adenocarcinoma. Transl Oncol. 2020;13:100820.
Li C, Jiang C, Gong J, Wu X, Luo Y, Sun G. A CT-based logistic regression model to predict spread through air space in lung adenocarcinoma. Quant Imaging Med Surg. 2020;10:1984.
Chen L-W, Lin M-W, Hsieh M-S, et al. Radiomic values from high-grade subtypes to predict spread through air spaces in lung adenocarcinoma. Ann Thorac Surg. 2022;114:999–1006.
Onozato Y, Nakajima T, Yokota H, et al. Radiomics is feasible for prediction of spread through air spaces in patients with nonsmall cell lung cancer. Sci Rep. 2021;11:1–10.
Tao J, Liang C, Yin K, et al. 3D convolutional neural network model from contrast-enhanced CT to predict spread through air spaces in non-small cell lung cancer. Diagn Interv Imaging. 2022;103:535–44.
Katsumata S, Aokage K, Nakasone S, et al. Radiologic criteria in predicting pathologic less invasive lung cancer according to TNM 8th edition. Clin Lung Cancer. 2019;20:e163–70.
Wu G, Woodruff HC, Shen J, et al. Diagnosis of invasive lung adenocarcinoma based on chest CT radiomic features of part-solid pulmonary nodules: a multicenter study. Radiology. 2020;297:451–8.
Amin MB, Edge SB. AJCC cancer staging manual. Springer; 2017.
Chen L-W, Yang S-M, Chuang C-C, et al. Solid attenuation components attention deep-learning model to predict micropapillary and solid patterns in lung adenocarcinomas on computed tomography. Ann Surg Oncol. 2022;29:7473–82.
Acknowledgment
We would like to thank Ms. Yu-Hsuan Hu and Ms. Wei-Ying Huang for data collection, analysis and project administration. We also would like to thank Dr. Xu-Heng Chiang, Dr. Chao-Wen Lu, Dr. Tzu-Ning Kao and Dr. Hsin-Ying Lee for their interpretation of CT Images. This work was supported by grants from the Ministry of Science and Technology, Taipei, Taiwan (Grant Nos. MOST 111-2221-E-002-070 and 111-2811-E-002-004-MY2) and by the National Taiwan University Hospital, Taipei, Taiwan (Grant No. NTUH111-S0199). We thank Ms. Yu-Hsuan Hu and Ms. Wei-Ying Huang for data collection and analysis as well as project administration. We also thank Dr. Xu-Heng Chiang, Dr. Chao-Wen Lu, Dr. Tzu-Ning Kao, and Dr. Hsin-Ying Lee for their interpretation of CT images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflicts of interest.
Ethical Approval
This study was approved by the Institutional Review Board of the National Taiwan University Hospital (approval number: 202207035RIND) and National Taiwan University Hospital Hsin-Chu Branch (Approval Number: 107-033-E).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, MW., Chen, LW., Yang, SM. et al. CT-Based Deep-Learning Model for Spread-Through-Air-Spaces Prediction in Ground Glass-Predominant Lung Adenocarcinoma. Ann Surg Oncol 31, 1536–1545 (2024). https://doi.org/10.1245/s10434-023-14565-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14565-2