Abstract
Background
The clinical role of peritoneal lavage cytology (CY) in pancreatic ductal adenocarcinoma (PDAC) remains controversial, partly due to its low sensitivity. This study aimed to develop a new biomarker, defined as peritoneal lavage tumor DNA (ptDNA), using DNAs extracted from peritoneal lavage samples from patients with PDAC.
Methods
Samples were collected intraoperatively from 89 PDAC patients who underwent pancreatectomy between 2012 and 2017. Droplet digital polymerase chain reaction (PCR) was used to measure ptDNA for detection of KRAS mutations. The ptDNA status and clinical characteristics were retrospectively evaluated.
Results
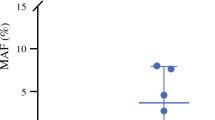
Positive ptDNA was found in 41 patients, including all 9 patients positive for CY (CY+) and 32 patients negative for CY (CY−). The mutant allele frequency was significantly higher in the CY+ patients than in the CY− patients. The disease-free survival (DFS) and overall survival (OS) were significantly poorer in the high-ptDNA group than in the low-ptDNA group (median DFS, 11.0 vs. 18.8 months; p = 0.007; median OS, 28.7 vs not reached; p = 0.001). The survival curves of DFS and OS in the CY+ group were almost equal to those in the CY− and high-ptDNA group. In a multivariable analysis, ptDNA was an independent predictive factor for DFS (p = 0.025) and OS (p = 0.047). The estimated cumulative incidence of peritoneal recurrence was 45.5% in the high-ptDNA group. The ptDNA biomarker had a much higher sensitivity for peritoneal recurrence than CY, whereas CY had higher specificity.
Conclusions
As a promising biomarker, ptDNA may predict poor prognosis and peritoneal recurrence in PDAC, resolving the controversy surrounding CY.



Similar content being viewed by others
References
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21.
Mokdad AA, Minter RM, Zhu H, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: a propensity score-matched analysis. J Clin Oncol. 2017;35:515–22.
Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388:248–57.
Suenaga M, Fujii T, Kanda M, et al. Pattern of first recurrent lesions in pancreatic cancer: hepatic relapse is associated with dismal prognosis and portal vein invasion. Hepatogastroenterology. 2014;61:1756–61.
Kodera Y, Yamamura Y, Shimizu Y, et al. Peritoneal washing cytology: prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol. 1999;72:60–4; discussion 4–5.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
Tempero MA, Arnoletti JP, Behrman S, et al. Pancreatic adenocarcinoma. J Natl Compr Canc Netw. 2010;8:972–1017.
Sobin L, Gospodarowicz M, Wittekind C (eds). International Union against Cancer (UICC) TNM Classification of Malignant Tumours. 7th ed. Wiley Blackwell, New York, 2011.
Japan Pancreas Society. Classification of Pancreatic Carcinoma. 4th English ed. Kanehara, Tokyo, Japan, 2017.
Yamada S, Fujii T, Kanda M, et al. Value of peritoneal cytology in potentially resectable pancreatic cancer. Br J Surg. 2013;100:1791–6.
Satoi S, Murakami Y, Motoi F, et al. Reappraisal of peritoneal washing cytology in 984 patients with pancreatic ductal adenocarcinoma who underwent margin-negative resection. J Gastrointest Surg. 2015;19:6–14.
Virgilio E, Giarnieri E, Giovagnoli MR, et al. Gastric cancer cells in peritoneal lavage fluid: a systematic review comparing cytological with molecular detection for diagnosis of peritoneal metastases and prediction of peritoneal recurrences. Anticancer Res. 2018;38:1255–62.
Vogelstein B, Kinzler KW. Digital PCR. Proc Natl Acad Sci U S A. 1999;96:9236–41.
Biankin AV, Waddell N, Kassahn KS, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405.
Kanda M, Matthaei H, Wu J, et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730–3 e9.
Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:1028–61.
Ikeda M, Okusaka T, Ito Y, et al. A phase I trial of S-1 with concurrent radiotherapy for locally advanced pancreatic cancer. Br J Cancer. 2007;96:1650–5.
Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703.
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25.
Fujii T, Sugimoto H, Yamada S, et al. Modified Blumgart anastomosis for pancreaticojejunostomy: technical improvement in matched historical control study. J Gastrointest Surg. 2014;18:1108–15.
Fujii T, Nakao A, Yamada S, et al. Vein resections > 3 cm during pancreatectomy are associated with poor 1-year patency rates. Surgery. 2015;157:708–15.
Suenaga M, Sadakari Y, Almario JA, et al. Using an endoscopic distal cap to collect pancreatic fluid from the ampulla (with video). Gastrointest Endosc. 2017;86:1152–6 e2.
Yamada S, Takeda S, Fujii T, et al. Clinical implications of peritoneal cytology in potentially resectable pancreatic cancer: positive peritoneal cytology may not confer an adverse prognosis. Ann Surg. 2007;246:254–8.
Yoshioka R, Saiura A, Koga R, et al. The implications of positive peritoneal lavage cytology in potentially resectable pancreatic cancer. World J Surg. 2012;36:2187–91.
Ferrone CR, Haas B, Tang L, et al. The influence of positive peritoneal cytology on survival in patients with pancreatic adenocarcinoma. J Gastrointest Surg. 2006;10:1347–53.
Abe T, Ohuchida K, Endo S, et al. Clinical importance of intraoperative peritoneal cytology in patients with pancreatic cancer. Surgery. 2017;161:951–8.
Kodera Y, Nakanishi H, Ito S, et al. Quantitative detection of disseminated cancer cells in the greater omentum of gastric carcinoma patients with real-time RT-PCR: a comparison with peritoneal lavage cytology. Gastric Cancer. 2002;5:69–76.
Sergeant G, Roskams T, van Pelt J, Houtmeyers F, Aerts R, Topal B. Perioperative cancer cell dissemination detected with a real-time RT-PCR assay for EpCAM is not associated with worse prognosis in pancreatic ductal adenocarcinoma. BMC Cancer. 2011;11:47.
Hoskovec D, Varga J, Konecna E, Antos F. Levels of CEA and CA19-9 in the sera and peritoneal cavity in patients with gastric and pancreatic cancers. Acta Cir Bras. 2012;27:410–6.
Suenaga M, Yu J, Shindo K, et al. Pancreatic juice mutation concentrations can help predict the grade of dysplasia in patients undergoing pancreatic surveillance. Clin Cancer Res. 2018;24:2963–74.
Satoi S, Yanagimoto H, Yamamoto T, et al. A clinical role of staging laparoscopy in patients with radiographically defined locally advanced pancreatic ductal adenocarcinoma. World J Surg Oncol. 2016;14:14.
Ishigami H, Kitayama J, Kaisaki S, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010;21:67–70.
Satoi S, Fujii T, Yanagimoto H, et al. Multicenter phase II study of intravenous and intraperitoneal paclitaxel with S-1 for pancreatic ductal adenocarcinoma patients with peritoneal metastasis. Ann Surg. 2017;265:397–401.
Mikula-Pietrasik J, Uruski P, Tykarski A, Ksiazek K. The peritoneal “soil” for a cancerous “seed”: a comprehensive review of the pathogenesis of intraperitoneal cancer metastases. Cell Mol Life Sci. 2018;75:509–25.
Acknowledgments
This work was supported by the Aichi Cancer Research Foundation. The authors thank Dr. Akiko Kada, a biostatistician at Nagoya Medical Center, Nagoya, Japan, for her kind advice. They also thank Yoko Nishikawa and Hisae Mizuno for their excellent assistance in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suenaga, M., Fujii, T., Yamada, S. et al. Peritoneal Lavage Tumor DNA as a Novel Biomarker for Predicting Peritoneal Recurrence in Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol 28, 2277–2286 (2021). https://doi.org/10.1245/s10434-020-08990-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08990-w