Abstract
The kinetics and optimization of oil extraction from gmelina seed was studied. The effect of various process variables such as temperature, time, volume of solvent, particle size, and their interaction on oil yield was investigated. A predictive model describing the oil yield in terms process variables was derived from multiple regression analysis. Optimum yield of 49.90 % was predicted at extraction temperature of 60 °C, extraction time of 60 min, seed particle size of 150 µm and 150 ml volume of solvent, for the process. It was found that oil yield increased with increase in temperature, time and volume of solvent but decreased with increase in seed particle size. The extract was analyzed to examine its physico-chemical properties (acid value, iodine value, peroxide value, viscosity, saponification value, moisture and ash content, refractive index, smoke, flash and fire points, and specific gravity) and structural elucidation by standard methods and instrumental techniques. Results revealed that the oil is not edible but find potential in biodiesel production. The kinetic study showed that the extraction process follows a second order mechanism with a rate constant of 1.26 × 1011 (dm3 mol−1)3 S−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rapidly growing global demand for energy and industrial raw materials from crude oil and the consequent depletion of crude oil reserves in addition to adverse environmental concerns and unstable nature of the international market make imperative the need to explore alternative sources of fuel and industrial raw materials. There has been an increase in the world production of oil seeds over the last 30 years. This would appear to be related to the increasing demand for oil seed products and by-products. Most seeds are cultivated primarily for their oil and meal. The oil from most seeds can be used for edible purposes (example is fluted pumpkin seed oil, soya bean seed oil) and about 80 % of the world production of vegetable oil is for human consumption while the remaining 20 % is shared between animals and chemical process industries [1]. As a result of the extensive demand of oil for consumption and industrial uses, analysis of many oils has been carried out. Most vegetable seed oils find wide applications in the production of soaps, paints, varnishes, lacquers, lubricants, hydraulic fluids, printing inks, dyes, pesticides, and insecticides [2–5]. Also, with increase in petroleum prices and uncertainties surrounding petroleum availability, vegetable oil can be employed as an alternative fuel and for biodiesel production which aims to overcome energy crisis problem [6]. Due to the increasing applications of vegetable oils, a number of seed oils have been characterized but the vast majority have not been adequately evaluated and this is particularly valid for gmelina, which falls into this group of under-utilized species of plant. The ability of a particular oil seed to fit into the growing industry depends on its utilization potential, rate of production, and availability of processing technology [7].
Gmelina arborea is a fast growing tree, which grows on different localities and prefers moist fertile valleys, they attain moderate to large height up to 40 m and 140 cm in diameter [8]. It is occurring naturally throughout greater part of India at altitudes up to 1,500 m. It also occurs naturally in Myanmar, Thailand, Laos, Cambodia, Vietnam, and in southern provinces of China, and has been planted extensively in Sierra Leone, Nigeria and Malaysia [9].
A lot of researches are being carried to find alternative ways of producing oil for process industries and for food industry. It has been found that almost all the seeds contain oil, hence these gives ground for other researchers to consider studies on the possible uses of other oil producing substances found in people’s everyday lives. There are various ways of extracting oil from oilseeds but solvent extraction has been reported to be most efficient techniques [10]. Now, gmelina seeds are already proven to produce oil [11], this fact itself is already useful information for researchers who seek to find alternative sources of oil. The ability of the oil to fit depends on its constituents, its composition, the rate of production, and availability of the processing technology. The study of these constituents is important for their effective uses. Recently, Sangay et al. [6], reported that a biodiesel produced from gmelina seed oil met all the necessary biodiesel standard of ASTM D6751 and EN 14214. Gmelina seed oil have been found to be a sustainable material for biodiesel and alkyd resin synthesis in terms of its availability, renewability, and biodegradability. Gmelina based biodiesel has been developed keeping two criteria in mind; the biodiesel met all the technical and industrial standards and, met all the ecologically relevant standards [6, 12].
The solvent extraction process must be carried out within a predetermined space of the controllable factors that are known to have considerable effects on the system response i.e. oil yield. The characterization (or screening) of these major contributing variables to the process response was achieved through design-of-experiment (DOE) and analysis of variance (ANOVA) while the control and optimization tasks were accomplished using the well-known response surface methodology (RSM).
The central focus of this study therefore, is to: explore the most significant factors which influence the overall efficiency of gmelina seed oil extraction process; characterize their effects and predict the possible route(s) to a desired optimal; characterize the extract for structural establishment and physico-chemical properties; evaluate a suitable kinetics mechanism and rate constant for the extraction process. Gmelina seed has been found most suitable in that; it is not edible, widely available, will not interfere in the food chain and can stand out as sustainable material for energy. Overall, this new solvent extraction design will guarantee increased overall process efficiency through;
-
1
Lower investment costs (more efficient and easy to execute).
-
2.
Lower operating costs (operating at optimal conditions of the process variables).
RSM has been widely adopted in industries such as drug and food industry, chemical and biological processes, for the purpose of either producing high quality products or operating the process in a more economical manner and ensuring that the process operates in a more stable and reliable way [13]. It has also been successfully applied to different processes for achieving its optimization using experimental designs [14–20].
Kinetic modelling
A relevant kinetic data are required to analyze and design an extraction process especially in industrial scale. In these experiments, mass transfer kinetic model was proposed to represent our experimental data because there is no reaction between gmelina seed oil with the organic solvent, n-hexane. For this model, the main mechanism which controls the rate of gmelina seed oil extraction was assumed to be the mass transfer of gmelina seed oil from gmelina seed (solid) to bulk liquid n-hexane. Mass transfer rate of the oil from gmelina seed to organic solvents can be written as;
where dW A /dt is the mass transfer rate of gmelina seed oil (g/s), C A and C Ai are the concentration of gmelina seed oil in bulk liquid (organic solvents) at time t (g L−1) and at equilibrium (g L−1), respectively. K is mass transfer coefficient and A is surface area for mass transfer process. Since the extraction was taken in batch process and its volume was kept constant during process, therefore
where k · a is volumetric mass transfer coefficient. Applying boundary conditions as follows:
-
1.
At the beginning of extraction process (t = 0), the mass of gmelina seed oil in bulk liquid is zero, W A = 0.
-
2.
At any time t, mass of gmelina seed oil in bulk liquid is W A = W Ai [10].
On integration, Eq. (5) gives;
Equation (6) can be rearranged in terms of yield per mass of gmelina seed to give:
where Y A and iY Ai are yield of gmelina seed oil in bulk liquid at time t and at equilibrium per mass of gmelina seed.
If a second-order rate law is considered, the rate of dissolution for the oil contained in the solid to solution can be described as:
where k = the second-order extraction rate constant (L g−1 min−1), C s = the extraction capacity (concentration of oil at saturation in g L−1), C t = the concentration of oil in the solution at any time (g L−1), t (min).
By considering the boundary condition t = 0 − t and C t = 0 − C t , the integrated rate law for a second-order extraction was obtained:
Linear form of the Eq. (9) would be:
The extraction rate can be written as:
As t → 0, the initial extraction rate, h, can be written as:
Rearranging Eq. (12), the concentration of oil at any time can be obtained as:
The initial extraction rate, h, the extraction capacity, C s and the second-order extraction constant, k, can be calculated experimentally by plotting t/C t vs. t.
The influence of temperature on the rate constant was determined by fitting k to Arrhenius equation,
Using plots of ln k as a function of 1/T
Materials and methods
Materials
The gmelina fruits were collected locally from a forest in ministry of forest reserve, Anambra State in Nigeria. It was soaked in water for 8 days so as to easily separate the fruit pulp from the seed (de-pulp). The seeds were sun-dried and crushed mechanically using corona blender; the crushed samples were then separated into different particle sizes using laboratory test sieves (150, 300, 600, 850 µm and 1 mm). The samples were then dried using the mermmet oven, stored in air tight containers and were labeled adequately.
Ethanol, sodium hydroxide, potassium hydroxide, and hydrochloric acid were obtained from BDH chemical ltd., Poole, England. Petroleum ether, diethyl ether, phenolphthalein indicator, glacial acetic acid, chloroform, distilled water, carbon tetrachloride, wij’s iodide solution were purchased from Merck chemicals, Germany. Potassium iodide (KI) and sodium thiosulphate were obtained from M&B, England. The organic solvent used for the oil extraction was n-hexane. All the reagents were commercial grade and were used without further purification.
Instruments and methods
The analysis of the oil was performed with a Thermo Finnigan Trace GC/Trace DSQ/A1300, (E.I Quadropole) equipped with a SGE-BPX5 MS fused silica capillary column (film thickness 0.25 µm) for GC–MS detection, and an electron ionization system with ionization energy of 700 eV was used. Carrier gas was helium at a flow rate of 10 ml min−1 injector and MS transfer line temperatures were set at 220 and 290 °C, respectively. The oven temperature was programmed from 50 to 150 °C at 3 °C min−1, then held isothermal for 100 min, and raised to 250 °C at 10 °C min−1. Diluted samples (1/100, v/v, in methylene chloride) of 1.00 µL were injected manually in the slitless mode. The identification of individual components was based on the comparison of their relative retention times with those of authentic samples on SGE-BPX5 capillary column, and by matching their mass spectral of peaks with those obtained from authentic samples and/or the Wiley 7N and TRLIB libraries spectra and published data. The chemical compositions of oil were also confirmed by SHIMADZU FTIR-84008. Viscosity was determined by Brookfield viscometer, RVT Model (#Spindle 3, RPM 20). The physico-chemical properties of the extract were determined by standard methods (ASTM, 1973).
Extraction of oil from gmelina
Ten grams of grinded meal was extracted with n-hexane. The extraction temperature was varied from 20 °C to boiling point of the solvent while the reaction time was varied between 5 and 60 min. The solvent to solid ratio was investigated from 2:1 to 5:1 and particle size was varied from 150 to 1,000 µm. At the end of the extraction, the micelle was filtered using a vacuum filtration (Millipore glass base and funnel) to remove suspended solids. Subsequently, the solvent was separated from the oil using rotary vacuum evaporator (Laborota 4000) and was collected in the receiving flask. The oil which was remained in the sample flask was weighed after the process was completed. The percentage of extracted oil was calculated by dividing the amount of obtained oil by the amount of the seeds multiplied by 100. The percentage oil yield was calculated using the expression below:
where, Y is the oil yield (%), W0 is the weight of pure oil extracted (g) and W is the weight of the sample of gmelina seed used in the experiment.
Formulation of design matrix
Preliminary data analysis conducted using steepest ascent method shows that curvature effects is possible. In view of curvature, a reduced order quadratic model (ROQM) was fitted over the resulting data as suggested in Eq. (14)
Equation (14) serves as the global predictive equation from which specific solution may be derived. The determination of the unknown coefficients of β0, β i , β ij , and δ ij is accomplished via regression analysis implemented on the statistical analysis software Design-Expert Version 9.1.7.1 trial from the Stat-Ease Inc. using the data recorded from the investigation.
The determination of unknown coefficient of Eq. (14) applies the design matrix of Table 2 formulated by judicious transformation of the actual values of the four control variables at various levels over which the experiments were executed to their coded equivalents using −1 and +1 notations to designate low and high level factor setting and ‘±α’ and ‘0’ for axial and centre points, respectively. The coded values of the independent variables for the design of the experiment for gmelina extraction process are given in Table 1. For statistical analysis, the variables \( X_{i} {\mkern 1mu} (i = 1,2, \ldots ,4) \) were coded A, B, C and D. The data given in Table 1 were used to formulate a global design matrix of Table 2 below from which further analyses were derived. \( Y \) is the response (oil yield) across the various experimental runs. Equation (14) was fitted to the experimental data presented in Table 2 to obtain the final predictive equation for the reaction progress in terms of the coded variables.
Process analysis
The extraction process was analyzed for the different interaction effects of the process variables on the responses based on solutions derived from Eq. (14) at some assumed experimental conditions so as to reveal possible routes to optimum yield. The popular surface response methodology was suitably utilized for this exercise. The design expert analysis and optimization tool functions provided all the necessary computational assistance. The results were presented as response surface contours.
Results and discussion
Process/statistical analysis
ANOVA for gmelina extraction process
The ANOVA results derived from the predictive model show that the main linear effects due to individual control factors such as particle size (x1), time (x2), solvent ratio (x3), and temperature (x4) coded as A, B, and C and D, respectively, are all significant process variables, with the observed P values <0.05 in the numerical analysis. This is equally true with the linear interaction effects between particle size and temperature (AD), seed particle size and solvent ratio (AC), and temperature and solvent ratio (CD). The quadratic effect of temperature, denoted by D2 is significant. The data obtained for specific investigation were refitted with a modified model obtained by excluding the non-significant variables from the general predictive equation and the results of statistical analysis obtained for the sequence of experimentations are summarized in Table 3. The coefficients of determination R2 values of 0.9523 obtained for the gmelina seed oil extraction process show that more than 95 % of the overall system variability can be explained by the empirical models of Eq. (14) which is specific case of the general predictive equation derived for the investigation from the multivariate regression analyses implemented on design expert.
where Y is the predicted value of the dependent variable (oil yield). The coefficients of A, B, C and D are the main linear effects of the independent process variables: seed particle size (x1), time (x2), solvent ratio (x3), and temperature (x4) respectively, in coded units. AD, DB, and CB represent the linear interaction effects between particle size/temperature, temperature/time, and solvent ratio/time, respectively. A2, B2,C2 and D2 are the quadratic effects of the respective process variables. The “pred R-squared” of 0.7253 is in reasonable agreement with the “adj R-squared” of 0.9078. “adeq precision” measures the signal to noise ratio. A ratio greater than 4 is desirable. The design ratio of 16.924 indicates an adequate signal. This model can be used to navigate the design space and the model F value of 21.40 further indicates that the model is significant. There is only a 0.01 % probability that the “model F value” this large could occur due to noise. P value less than 0.05 indicates model terms are significant.
3-D response surface plots for the optimization process
The 3-D response surface plots are graphical representations of the interactive effects of any two variables. The nature of the response surface curves shows the interaction between the variables. An elliptical shape of the curve indicates good interaction of the two variables and circular shape indicates no interaction between the variables. The 3-D response surface plots shown in Figs. 1, 2, 3, 4, 5 and 6 for the chosen model equation shows the relationship between the independent and the dependent variables. From Fig. 6, the response surface indicates that the percentage yield of oil increases as temperature and solvent composition increases to optimum condition while further increase leads to decrease of percentage yield of oil. In addition, there was mutual interaction between the temperature and solvent composition. The highest percentage oil yield was obtained when 153 ml of solvent was used. This is in accordance with the result obtained by Meziance and Kadi [27] that studied kinetics and thermodynamics of oil extraction from olive cake. They reported that the positive effect of volume of solvent on oil yield was as a result of increase in the concentration driving force as volume of solvent increases. It was also as a result of increased washing of the oil extracted, away from the particle surface by the solvent as a result of increased volume. The increase in oil yield became less significant at 125 ml because 125 ml hexane was sufficient to bring the oil solute to equilibrium. Similarly, the oil yield increased as the temperature increased from 20 to 60 °C (from the same figure). The highest oil yield was obtained at 60 °C. Hickox [28] also reported increase in oil yield with increase in temperature for cotton seed oil. The positive effect of temperature on oil yield is as a result of rupturing of oil cell walls which now creates a void which serves as migratory space for the contents of the oil bearing cells [29]. Temperature influences oil yield and higher extraction is achieved by increasing the temperature, which lowers the viscosity of the oil, releases oil from the intact cells and draws out moisture. Figures 1, 2 and 3 display the responses for the interactive factors; time (x2) vs seed particle size (x1), solvent ratio (x3) vs seed particle size (x1), and temperature (x4) vs particle size (x1), respectively. The 3-D response surface plots shown in Figs. 1, 3 and 4 shows drop in percentage oil yield when seed particle size increases; even at highest setting of temperature (60 °C), time (60 min), solvent ratio (5.0). The negative effect of seed particle size on oil yield could be attributed to the fact that smaller particles have larger amount of surface area coupled with increased number of ruptured cells resulting in a high oil concentration at the particle surface and low or little diffusion into the particles surface [30]. Sayyar et al. [1], while investigating the extraction of oil from Jatropha seed, suggested also that large particles have smaller amount of surface areas and are more resistant to intrusion of solvent and oil diffusion. Therefore, small amount of oil will be carried from inside the large particles to the surrounding solution. The quadratic effect of temperature (D) is visibly evident from the smooth curve in the response surface plots (see Fig. 3, 6). Dragon et al. [30], reported 46 % oil yield at extraction time of 120 min for gmelina seed using solvent extraction method while the current research recorded 49.50 % oil yield at extraction time of 60 min. Overall, given the long operational time, the earlier report may not be economically advantageous in terms of energy savings.
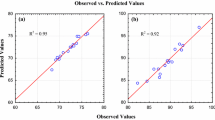
The normal plot of residuals (Fig. 7) was used to check whether the points will follow a straight line in which we concluded that the residuals follow a normal distribution. Hence, from the figures it is seen that the points were closely distributed to the straight line of the plot, it confirms the good relationship between the experimental values and the predicted values of the response though some small scatter like an “S” shape is always expected. These plots equally confirm that the selected model was adequate in predicting the response variables in the experimental values.
Kinetic study on extraction of gmelina seed oil
Linearization of the Arrhenius law as shown below gives the value of the activation energy (E) and the temperature independent factor (A) from ln(k) against 1/T plot: \( \ln \left( k \right) = \ln \left( A \right) + \left( {\frac{ - E}{R}} \right)\frac{1}{T} \)
The plot of ln(k) against 1/T gives ln(A) as the slope and \( \left( {\frac{ - E}{R}} \right) \) as the intercept. A plot of ln(dY/dt) versus ln Y was found to be linear. A second order kinetics was obtained from the slope of the straight line as shown in Fig. 8. The reaction rate constant was determined from the intercept as 1.26 × 1011 (dm3 mol−1)3 S−1 (Fig. 9).
The positive value of enthalpy change indicates that the process is endothermic and requires energy during process.
Characteristics of gmelina seed oil
The fatty acid composition of gmelina seed oil was analysed by gas chromatography mass-spectrometry (GC–MS). Table 4 and Fig. 10 show the fatty acids present in gmelina seed oil. GC–MS analysis of the oil showed abundance of palmitoleate (31.94 %wt) and arachidic acid (17.16 %wt). The most abundant unsaturated and saturated fatty acids were palmitoleate (31.94 %wt) and methyl stearate (14.22 %wt), respectively. The oil contains 50.86 % saturated fatty acid and 49.10 % unsaturated fatty acid. The results for the physico-chemical properties of the extracted oil are presented in Table 5. The physico-chemical analysis of the gmelina seed oil indicated physical state of the oil to be liquid and amber yellow at room temperature. The oil content of gmelina arborea seed was found to be 54.6 %wt. The oil content is significant and compares favourably with seed oil of other plants such as Hevea brasiliensis (51 %wt), Hematostaphis berter (54.5 %wt), Jatropha curcas (30–50 %wt), Sapindus mukorossi (51 %wt), Mellia azadirachta (33–45 %wt), and 55–65 % wt for Simarouba glauca [21]. On the basis of the oil content, gmelina arborea seed would be highly suitable economically for industrial applications, as any oil bearing seed that can produce up to 30 % oil are regarded as suitable [7]. The flash, fire, and smoke points were found to be 331.54, 386.12, and 176 °C respectively and were all above the 130 °C minimum ASTM D6751 and 120 °C EN 14214 Biodiesel (B100) Specifications and therefore pose very negligible or no risk of fire outbreaks in case of accidents (when used as biodiesel) or spills. A value of 1.441 was obtained for the refractive index. The refractive index value obtained falls within the range (1.447–1.490) reported [22] for some other seed oils (1.480 for Telfairia occidentalis, 1.468 for Jatropha curcas, 1.47 for soybean oil and 1.47 for corn oil) which have myriad industrial applications. The specific gravity of gmelina seed oil was found to be 0.892 at 25 °C and this value is in the range found for other common oils. The SG value is also within the range (0.860–0.900) stipulated by EN 14214 for biodiesel [23]. Iodine value of 31.09 mleq g−1 was obtained. Iodine value measures the degree of unsaturation of a particular vegetable oil, oils with iodine value above 135 are classified as drying oil, those with iodine value 110–130 are classified as semi-drying oil and those with iodine value below 90 are non-drying oils. The iodine value of 31.09 mleq g−1 for gmelina seed oil is comparable to literature values of castor oils and olive oils which are both non-drying oils. The iodine value suggests the oil contains mainly saturated fatty acids, thus the oil is not suitable as alkyd resin for paint formulation or use as varnishes; it may, however, find uses in conjunction with amino resins as finishes for certain appliances, and in this case the oil may also act as plasticizers. The iodine value is also consistent with the corresponding total saturation of fatty acids (50.86 %) derived from Table 3, thus affirming the oil is largely consisting of saturated fatty acids. Peroxide value of 9 mleq g−1 was obtained for the oil. Peroxide value depends on a number of factors such as oxidation by oxygen, extraction methods, and storage. The low peroxide value suggests that gmelina seed oil is stable to oxidative degradation caused by over exposure to oxygen, heating and improper storage [24]. Saponification value of 32.258 mleq g−1 was obtained for the oil. The saponification value is very low when compared with the values for common oils; palm oil (196–205), groundnut oil (188–196), and corn oil (187–196) [25]. The low saponification value is as a result of the abundant long chain fatty acids (found in the oil) which have a relatively fewer number of carboxylic functional groups per unit mass of the oil as compared to short chain fatty acids. Thus the oil is not very suitable to be used for soap production. An acid value of 0.0336 mgKOH g−1 gives an indication of the amount of FFA present in the oil at the time of the test. The low acid value is an indication of good non-degraded state of the oil and is within limits for industrial useful oils. The FFA concentration of the oil was low (0.0168) which was consistent with low acid value observed. The low FFA also suggests low levels of hydrolytic and lipolytic activities in the oils. The 5 % total ash content is comparable to 4.56 % for Jatropha curcas [26] and it indicates the possible presence of abrasive solids, soluble metallic soaps, and silica residue in the seed. The ash content exceeds the ASTM standard of 0.02 maximum for biodiesel production. The moisture content of the oil (7.5 %) was very high, far exceeding the stipulated ASTM standard (0.05 %) for biodiesel production. This high moisture content creates problem in, reduces shelf life and may cause corrosion in internal combustion engine [15]. The structural organization of extracted gmelina nut oil was investigated by FTIR as shown in Fig. 11. In the spectrum of gmelina raw oil, 3,498.02 cm−1 correspond to the hydroxyl group (O–H) of the unsaturated fatty acid in the oil. The carboxyl group (C=O) is indicated at 1,646.3 cm−1 The straight chain of –CH– stretch in aliphatic compound is found at the band 2,924.18 cm−1. Alkene group (CH=CH) is attributed to the band of 3,206.78 cm.
Conclusion
This study has clearly demonstrated the applicability of RSM in selecting extraction conditions for gmelina oil from its seed. This approach has not only resulted in the maximum oil yield through solvent extraction, but has also guaranteed the fulfillment of the properties requirements of the gmelina oil. The optimum values for yield show that it is an economic source of oil, the low saponification value means that it is not a good ingredient for soap making. The oil is very saturated and can hardly be used for paint making but when combined with other substances it can be used as a finishing agent. Time, seed particle size, temperature, solvent volume, have various effects on the yield of oil. The validation experiments and their accompany quality characteristics were not significantly different from the simulated values at P < 0.05. From spectroscopic results, it can be concluded that gmelina seed oil can be used as a source of biodiesel due to its specific gravity and saturated nature. Moreover, as a non-drying oil, it can also find variant uses in the process industry mainly when combined with some resins and compounds.
References
Sayyar, S., Abidi, Z.Z., Yunus, R., Mohammed, A.: Extraction of oil from jatropha seeds, optimization and kinetics. Am. J. App. Sci. 6(7), 1390–1395 (2009)
Uzoh, C.F., Onukwuli, O.D., Odera, R.S., Ofochebe, S.: Optimization of polyesterification process for production of palm oil modified alkyd resin using response surface methodology. J. Environ. Chem. Eng. 1, 777–785 (2013)
Uzoh, C.F., Onukwuli, O. D., Odera, R. S., Nwabanne, J. T.: Physico-chemical study of palm kernel oil alkyd resin and optimization of its polyesterification process. Int. J. Chem Eng. (2013). doi:10.1016/j.fuproc.2010.10.002
Silva, G.F., Camargo, F.L., Ferreira, A.L.O.: Application of response surface methodology for optimization of biodiesel production by transesterification of soybean oil with ethanol. Fuel Process. Technol. (2010)
Hlaing, N.N., Oo, M.M.: Manufacture of alkyd resin from castor oil. World Acad. Sci. Eng. Technol. 48 (2008)
Sangay, B., Priyanka, B., Dibakar, O.D.: Gmelina arborea and Tarbernaemontana divaricate seed oils as non-edible feedstock for biodiesel production. Int. J. Chem. Tech. Res. 6(2), 1440–1445 (2014)
Okolie, P.N., Ajekwene, A.E., Uaboi, Egbeni: Extraction and characterization of oil from Jatropha curcas seed. World J. Agric. Sci. 8(4), 359–365 (2012)
Tewari, D.N.: A monograph on Gamari (Gmelina arborea Roxb.), p 125. International Book Distributors, Dehra Dun. (1995)
Adegbehin, J.O., Abayomi, J.O., Nwaigbo, L.B.: Gmelina arborea in Nigeria. Commonw. For. Rev. 67(2), 159–166 (1988)
Topallar, H., Gecgel, U.: Kinetics and thermodynamics of oil extraction from sunflower seeds in the presence of aqeous acidic hexane solution. Turk. J. Chem. 24, 247–253 (2000)
Adeyeye, A.: Composition of seed oils of gmelina arborea and teak tectora-grandis. Pak. J. Sci. Ind. Res. 34(9), p359 (1971)
http://www.dainet.de/fnr/ctvo/paint/2_workshop/ull.doc. Accessed 23 April 2014
Sudamalla, P., Saravanan, P., Matheswaran, M.: Optimization of operating parameters using response surface methodology for adsorption of crystal violet by activated carbon prepared from mango kernel. Sustain. Environ. Res. 22(1), 1–7 (2012)
Gunawan, E.R., Suhendra, D.: Four-factor response surface optimization of the enzymatic synthesis of wax ester from palm kernel oil. Indones. J. Chem. 8(1), 83–90 (2008)
Alam, M.Z., Muyibi, S.A., Toramae, J.: Statistical optimization of adsorption processes for removal of 2, 4-dichlorophenol by activated carbon derived from oil palm empty fruit bunches. J. Environ. Sci. China 19(6), 674–677 (2007)
Abali, Y., Colak, S., Yapici, S.: The optimization of the dissolution of phosphate rock with Cl2–SO2 gas mixture in aqueous medium. Hydrometallurgy 46, 27–35 (1997)
Narayana, S.K.V., King, P., Gopinadh, R., Sreelakshmi, V.: Response surface optimization of dye removal by using waste prawn shells. Int. J. Chem. Sci. Appl. 2(3), 186–193 (2011)
Umesh, G., Sud, D.: Optimization of process parameters for removal of Cr(VI) from aqueous solutions using modified sugarcane bagasse. E-J. Environ. Agric. Food. Chem. 4(6), 1150–1160 (2005)
Li, D., Park, K., Wu, Z., Guo, X.: Response surface design for nickel recovery from laterite by sulfation-roasting-leaching process. Trans. Nonferrous Met. Soc. China 20, 92–96 (2010)
Basumatary, S., Deka, Dinesh C., Deka, Dibakar C.: Composition of biodiesel from Gmelina arborea seed oil. Adv. Appl. Sci. Res. 3(5), 2745–2753 (2012)
Eckey, E.W.: Vegetable fats and oils. American Chemical Society Monograph Series no. 123. Reinhold Publishing Corporation, New York (1954)
Ejikeme, P.M., Anyaogu I.D., Ejikeme, C.L., Nwafor, N.P., Egbuonu, C.A., Ukogu, K., Ibemesie, J.A.: Catalysis in biodiesel by transesterification processes-an insight. J. Chem. 7(4), 1120–1132 (2010)
Ibeto, C.N., Okoye, C.O., Ofoelue, A.U.: Comparative study of the physicochemical characterization of some oil as potential feedstock for biodiesel production. ISRN Renew. Energy 2012, 1–5 (2012)
Cocks, L.V., Van Rede C.: Laboratory Handbook for Oil and Fat Analysts, pp. 3–37, 107–130. Academic Press, London, New York (1966)
Nayak, B.S., Patel, K.N.: Physicochemical characterization of seed and seed Oil of Jatropha curcas. Sains Malays. 39(6), 951–955 (2010)
Meziance S., Kadi H., Lamrous O.: Kinetics study of oil extraction from olive foot cake
Hickox, G.H.: Some factors affecting the hydraulic extraction of cotton seed oil. J. Am. Oil Chem. Soc. 30, 481–486 (1953)
Adeeko, K.A., Ayibola, O.O.: Processing factors affecting yield and quality of mechanically expressed groundnut oil. J. Agric. Eng. 45, 31–43 (1990)
Ebewele, R.O., Iyayi, A.F., Hymore, F.K.: Considerations of the extraction process and potential technical applications of Nigerian rubber seed oil. Int. J. Phys. Sci. 5(6), 826–831 (2010)
Dragon, R.G., Reyes, A.E.: Proceeding of the International Seminar on Chemistry, pp. 290–293, 30–31 October, Jatinagor (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Uzoh, C.F., Onukwuli, O.D. & Nwabanne, J.T. Characterization, kinetics and statistical screening analysis of gmelina seed oil extraction process. Mater Renew Sustain Energy 3, 38 (2014). https://doi.org/10.1007/s40243-014-0038-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40243-014-0038-1