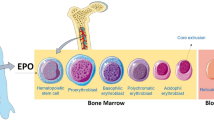
Abstract
The erythrocyte is a highly specialised cell with a limited metabolic repertoire. As an oxygen shuttle, it must continue to perform this essential task while exposed to a wide range of environments on each vascular circuit, and to a variety of xenobiotics across its lifetime. During this time, it must continuously ward off oxidant stress on the haeme iron, the globin chain and on other essential cellular molecules. Haemolysis, the acceleration of the normal turnover of senescent erythrocytes, follows severe and irreversible oxidant injury. A detailed understanding of the molecular mechanisms underlying oxidant injury and its reversal, and of the clinical and laboratory features of haemolysis is important to the medical toxicologist. This review will also briefly review glucose-6-phosphate deficiency, a common but heterogeneous range of enzyme-deficient states, which impairs the ability of the erythrocyte to respond to oxidant injury.












Similar content being viewed by others
References
Dreyfus C. Chronic hemolytic jaundice: a historical study. Bull New Engl Med Cent 1942; 4: 122–8
Packman CH. The spherocytic haemolytic anaemias. Br J Haematol 2001; 112(4): 888–99
Hunter W. Is pernicious anaemia a special disease? Practitioner 1888; 41: 81–103
Vanlair CF, Masius JB. De la microcythemie. Bull R Acad Med Belg 1871; 5: 515–613
Hayem G. Sur une variete particuliere d’ictere chronique. La Presse Medicale 1898; 6: 121–2
Widal F, Abrami P, Brule M. Pluralite d’origine des icteres hemolytiques (recherches cliniques et experimentales). Societe Medicale Des Hospitaux de Paris 1907; 24: 1354–67
Third, Fourth and Fifth reports of the committee for classification of the nomenclature of cells and diseases of the blood and bloodforming organs. Am J Clin Pathol 1950; 20: 562–79
Alving AS, Carson PE, Flanagan CL, et al. Enzymatic deficiency in primaquine-sensitive erythrocytes. Science 1956; 124(3220): 484–5
Piccinini G, Minetti G, Balduini C, et al. Oxidation state of glutathione and membrane proteins in human red cells of different age. Mech Ageing Dev 1995; 78(1): 15–26
Marschner JP, Seidlitz T, Rietbrock N. Effect of 2,3-diphosphoglycerate on O2-dissociation kinetics of hemoglobin and glycosylated hemoglobin using the stopped flow technique and an improved in vitro method for hemoglobin glycosylation. Int J Clin Pharmacol Ther 1994; 32(3): 116–21
Palek J. Introduction: red blood cell membrane proteins, their genes and mutations. Semin Hematol 1993; 30(1): 1–3
Seppi C, Castellana MA, Minetti G, et al. Evidence for membrane protein oxidation during in vivo aging of human erythrocytes. Mech Ageing Dev 1991; 57(3): 247–58
Schluter K, Drenckhahn D. Co-clustering of denatured hemoglobin with band 3: its role in binding of autoantibodies against band 3 to abnormal and aged erythrocytes. Proc Natl Acad Sci USA 1986; 83(16): 6137–41
Gow AJ, Luchsinger BP, Pawloski JR, et al. The oxyhemoglobin reaction of nitric oxide. Proc Natl Acad Sci USA 1999; 96(16): 9027–32
McMahon TJ, Moon RE, Luschinger BP, et al. Nitric oxide in the human respiratory cycle. Nat Med 2002; 8(7): 711–7
Reiter CD, Wang X, Tanus-Santos JE, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med 2002; 8(12): 1383–9
Mayhew TM, Mwamengele GL, Self TJ, et al. Stereological studies on red corpuscle size produce values different from those obtained using haematocritand model-based methods. Br J Haematol 1994; 86(2): 355–60
Lenard JG. A note on the shape of the erythrocyte. Bull Math Biol 1974; 36(1): 55–8
Bennett V. Spectrin-based membrane skeleton: a multipotential adaptor between plasma membrane and cytoplasm. [published erratum appears in Physiol Rev 1991 Jan; 71 (1)]. Physiol Rev 1990; 70(4): 1029–65
Murphy JR. Erythrocyte metabolism: II. Glucose metabolism and pathways. J Lab Clin Med 1960; 55: 286–302
Bloom GE, Zarkowsky HS. Heterogeneity of the enzymatic defect in congenital methemoglobinemia. N Engl J Med 1969; 281(17): 919–22
Canepa L, Ferraris AM, Miglino M, et al. Bound and unbound pyridine dinucleotides in normal and glucose-6-phosphate dehydrogenase-deficient erythrocytes. Biochim Biophys Acta 1991; 1074(1): 101–4
Micheli V, Simmonds HA, Bari M, et al. HPLC determination of oxidized and reduced pyridine coenzymes in human erythrocytes. Clin Chim Acta 1993; 220(1): 1–17
Kondo T, Dale GL, Beutler E. Studies on glutathione transport utilizing inside-out vesicles prepared from human erythrocytes. Biochim Biophys Acta 1981; 645(1): 132–6
Srivastava SK, Beutler E. Oxidized glutathione levels in erythrocytes of glucose-6-phosphate-dehydrogenase-deficient subjects. Lancet 1968; II(7558): 23–4
Schuster R, Holzhutter HG, Jacobasch G. Interrelations between glycolysis and the hexose monophosphate shunt in erythrocytes as studied on the basis of a mathematical model. Biosystems 1988; 22(1): 19–36
Minnich V, Smith MB, Brauner MJ, et al. Glutathione biosynthesis in human erythrocytes: I. identification of the enzymes of glutathione synthesis in hemolysates. J Clin Invest 1971; 50(3): 507–13
Srivastava SK, Beutler E. The transport of oxidized glutathione from human erythrocytes. J Biol Chem 1969; 244(1): 9–16
Telen MJ. Erythrocyte blood group antigens: polymorphisms of functionally important molecules. Semin Hematol 1996; 33(4): 302–14
Skandalakis PN, Colborn GL, Skandalakis LJ, et al. The surgical anatomy of the spleen. Surg Clin North Am 1993; 73(4): 747–68
Sass MD, Caruso CJ, Farhangi M. TPNH-methemoglobin reductase deficiency: a new red-cell enzyme defect. J Lab Clin Med 1967; 70(5): 760–7
Mansouri A. Methemoglobin reduction under near physiological conditions. Biochem Med Metab Biol 1989; 42(1): 43–51
Coleman MD, Coleman NA. Drug-induced methaemoglobinaemia: treatment issues. Drug Saf 1996; 14(6): 394–405
Bradberry SM. Occupational methaemoglobinaemia: mechanisms of production, features, diagnosis and management including the use of methylene blue. Toxicol Rev 2003; 22(1): 13–27
Riccio A, Vitagliano L, di Prisco G, et al. The crystal structure of a tetrameric hemoglobin in a partial hemichrome state. Proc Natl Acad Sci USA 2002; 99(15): 9801–6
Rifkind JM, Abugo O, Levy A, et al. Detection, formation, and relevance of hemichromes and hemochromes. Methods Enzymol 1994; 231: 449–80
Rachmilewitz EA. Denaturation of the normal and abnormal hemoglobin molecule. Semin Hematol 1974; 11(4): 441–62
Berzofsky JA, Peisach J, Blumberg WE. Sulfheme proteins: I. Optical and magnetic properties of sulfmyoglobin and its derivatives. J Biol Chem 1971; 246(10): 3367–77
Berzofsky JA, Peisach J, Horecker BL. Sulfheme proteins: IV. The stoichiometry of sulfur incorporation and the isolation of sulfhemin, the prosthetic group of sulfmyoglobin. J Biol Chem 1972; 247(12): 3783–91
Morell DB, Chang Y. The structure of the chromophore of sulphmyoglobin. Biochim Biophys Acta 1967; 136(1): 121–30
Chiu D, Lubin B. Oxidative hemoglobin denaturation and RBC destruction: the effect of heme on red cell membranes. Semin Hematol 1989; 26(2): 128–35
Cappellini MD, Tavazzi D, Duca L, et al. Metabolic indicators of oxidative stress correlate with haemichrome attachment to membrane, band 3 aggregation and erythrophagocytosis in beta-thalassaemia intermedia. Br J Haematol 1999; 104(3): 504–12
Ideguchi H. Effects of abnormal Hb on red cell membranes [in Japanese]. Rinsho Byori 1999; 47(3): 232–7
Hebbel RP, Eaton JW. Pathobiology of heme interaction with the erythrocyte membrane. Semin Hematol 1989; 26(2): 136–49
McCormick DJ, Atassi MZ. Hemoglobin binding with haptoglobin: delineation of the haptoglobin binding site on the alpha-chain of human hemoglobin. J Protein Chem 1990; 9(6): 735–42
Morgan WT, Muster P, Tatum F, et al. Identification of the histidine residues of hemopexin that coordinate with heme-iron and of a receptor-binding region. J Biol Chem 1993; 268(9): 6256–62
Smith A, Hunt RC. Hemopexin joins transferrin as representative members of a distinct class of receptor-mediated endocytic transport systems. Eur J Cell Biol 1990; 53(2): 234–45
Coburn RF, Williams WJ, Kahn SB. Endogenous carbon monoxide production in patients with hemolytic anemia. J Clin Invest 1966; 45(4): 460–8
International Committee for Standardization in Haematology, Expert Panel on Haemoglobinometry. Recommendations for reference method for haemoglobinometry in human blood (ICSH standard 1986) and specifications for international haemiglobincyanide reference preparation. 3rd ed. Clin Lab Haematol 1987; 9(1): 73–9
National Committee for Clinical Laboratory Standards. Reference and selected procedures for the quantitative determination of hemoglobin in blood. 2nd ed. NCCLS H15-A2. Villanova (PA): NCCLS, 1994
Wilkinson SP, McHugh P, Horsley S, et al. Arsine toxicity aboard the Asiafreighter. BMJ 1975; 3(5983): 559–63
McArthur JR, Hillman RS. Normal and abnormal peripheral blood cells. ASH slide bank. 3rd ed. Washington, DC: American Society of Hematology, 1990
Stevenson DK, Vreman HJ. Carbon monoxide and bilirubin production in neonates. Pediatrics 1997; 100 (2: Pt 1): 252–4
Petz LD. Drug-induced autoimmune hemolytic anemia. Transfus Med Rev 1993; 7(4): 242–54
Myint H, Copplestone JA, Orchard J, et al. Fludarabine-related autoimmune haemolytic anaemia in patients with chronic lymphocytic leukaemia. Br J Haematol 1995; 91(2): 341–4
Robak T, Blasinska-Morawiec M, Krykowski E, et al. Autoimmune haemolytic anaemia in patients with chronic lymphocytic leukaemia treated with 2-chlorodeoxyadenosine (cladribine). Eur J Haematol 1997; 58(2): 109–13
Bennett CL, Weinberg PD, Rozenberg-Ben-Dror K, et al. Thrombotic thrombocytopenic purpura associated with ticlopidine: a review of 60 cases. Ann Intern Med 1998; 128(7): 541–4
Tsai HM, Rice L, Sarode R, et al. Antibody inhibitors to von Willebrand factor metalloproteinase and increased binding of von Willebrand factor to platelets in ticlopidine-associated thrombotic thrombocytopenic purpura. Ann Intern Med 2000; 132(10): 794–9
Bennett CL, Connors JM, Carwile JM, et al. Thrombotic thrombocytopenic purpura associated with clopidogrel. N Engl J Med 2000; 342(24): 1773–7
Atkinson K, Biggs JC, Hayes J, et al. Cyclosporin A associated nephrotoxicity in the first 100 days after allogeneic bone marrow transplantation: three distinct syndromes. Br J Haematol 1983; 54(1): 59–67
Dzik WH, Georgi BA, Khettry U, et al. Cyclosporine-associated thrombotic thrombocytopenic purpura following liver transplantation: successful treatment with plasma exchange. Transplantation 1987; 44(4): 570–2
Shitrit D, Starobin D, Aravot D, et al. Tacrolimus-induced hemolytic uremic syndrome case presentation in a lung transplant recipient. Transplant Proc 2003; 35(2): 627–8
Mach-Pascual S, Samii K, Beris P. Microangiopathic hemolytic anemia complicating FK506 (tacrolimus) therapy. Am J Hematol 1996; 52(4): 310–2
Holman MJ, Gonwa TA, Cooper B, et al. FK506-associated thrombotic thrombocytopenic purpura. Transplantation 1993; 55(1): 205–6
Canpolat C, Pearson P, Jaffe N. Cisplatin-associated hemolytic uremic syndrome. Cancer 1994; 74(11): 3059–62
Gardner G, Mesler D, Gitelman HJ. Hemolytic uremic syndrome following cisplatin, bleomycin, and vincristine chemotherapy: a report of a case and a review of the literature. Ren Fail 1989; 11(2-3): 133–7
Murgo AJ. Thrombotic microangiopathy in the cancer patient including those induced by chemotherapeutic agents. Semin Hematol 1987; 24(3): 161–77
Necheles TF, Steinberg MH, Cameron D. Erythrocyte glutathione-peroxidase deficiency. Br J Haematol 1970; 19(5): 605–12
Yoo D, Lessin LS. Drug-associated ‘bite cell’ hemolytic anemia. Am J Med 1992; 92(3): 243–8
Lee E, Boorse R, Marcinczyk M. Methemoglobinemia secondary to benzocaine topical anesthetic. Surg Laparosc Endosc 1996; 6(6): 492–3
Ferraro-Borgida MJ, Mulhern SA, DeMeo MO, et al. Methemoglobinemia from perineal application of an anesthetic cream. Ann Emerg Med 1996; 27(6): 785–8
Cote MA, Lyonnais J, Leblond PF. Acute Heinz-body anemia due to severe cresol poisoning: successful treatment with erythrocytapheresis. CMAJ 1984; 130(10): 1319–22
Jollow DJ, Bradshaw TP, McMillan DC. Dapsone-induced hemolytic anemia. Drug Metab Rev 1995; 27(1–2): 107–24
Spooren AA, Evelo CT. Hydroxylamine treatment increases glutathione-protein and protein-protein binding in human erythrocytes. Blood Cells Mol Dis 1997; 23(3): 323–36
Sills MR, Zinkham WH. Methylene blue-induced Heinz body hemolytic anemia. Arch Pediatr Adolesc Med 1994; 148(3): 306–10
Brandes JC, Bufill JA, Pisciotta AV. Amyl nitrite-induced hemolytic anemia. Am J Med 1989; 86(2): 252–4
Bogart L, Bonsignore J, Carvalho A. Massive hemolysis following inhalation of volatile nitrites. Am J Hematol 1986; 22(3): 327–9
Romeril KR, Concannon AJ. Heinz body haemolytic anaemia after sniffing volatile nitrites. Med J Aust 1981; 1(6): 302–3
Beaupre SR, Schiffman FJ. Rush hemolysis: a ‘bite-cell’ hemolytic anemia associated with volatile liquid nitrite use. Arch Fam Med 1994; 3(6): 545–8
Todisco V, Lamour J, Finberg L. Hemolysis from exposure to naphthalene mothballs. N Engl J Med 1991; 325(23): 1660–1
Larkin EC, Williams WT, Ulvedal F. Human hematologic reesponses to 4 hr of isobaric hyperoxic exposure (100 per cent oxygen at 760mm Hg). J Appl Physiol 1973; 34(4): 417–21
Mengel CE, Kann Jr HE, Heyman A, et al. Effects of in vivo hyperoxia on erythrocytes: II. Hemolysis in a human after exposure to oxygen under high pressure. Blood 1965; 25: 822–9
Millar J, Peloquin R, De Leeuw NK. Phenacetin-induced hemolytic anemia. CMAJ 1972; 106(7): 770–5
Nathan DM, Siegel AJ, Bunn HF. Acute methemoglobinemia and hemolytic anemia with phenazopyridine: possible relation to acute renal failure. Arch Intern Med 1977; 137(11): 1636–8
Fincher ME, Campbell HT. Methemoglobinemia and hemolytic anemia after phenazopyridine hydrochloride (pyridium) administration in end-stage renal disease. South Med J 1989; 82(3): 372–4
Maloisel F, Kurtz JE, Andres E, et al. Platin salts-induced hemolytic anemia: cisplatin- and the first case of carboplatin-induced hemolysis. Anticancer Drugs 1995; 6(2): 324–6
Ward PC, Schwartz BS, White JG. Heinz-body anemia: ‘bite cell’ variant a light and electron microscopic study. Am J Hematol 1983; 15(2): 135–46
Klimecki WT, Carter DE. Arsine toxicity: chemical and mechanistic implications. J Toxicol Environ Health 1995; 46(4): 399–409
Kleinfeld MJ. Arsine poisoning. J Occup Med 1980; 22(12): 820–1
Fowler BA, Weissberg JB. Arsine poisoning. N Engl J Med 1974; 291(22): 1171–4
Jenkins GC, Ind JE, Kazantzis G, et al. Arsine poisoning: massive haemolysis with minimal impairment of renal function. BMJ 1965; 5453: 78–80
Hatlelid KM, Brailsford C, Carter DE. Reactions of arsine with hemoglobin. J Toxicol Environ Health 1996; 47(2): 145–57
Rael LT, Ayala-Fierro F, Carter DE. The effects of sulfur, thiol, and thiol inhibitor compounds on arsine-induced toxicity in the human erythrocyte membrane. Toxicol Sci 2000; 55(2): 468–77
Chuttani HK, Gupta PS, Gulati S, et al. Acute copper sulfate poisoning. Am J Med 1965; 39(5): 849–54
Fairbanks VF. Copper sulfate-induced hemolytic anemia: inhibition of glucose-6-phosphate dehydrogenase and other possible etiologic mechanisms. Arch Intern Med 1967; 120(4): 428–32
Sontz E, Schwieger J. The ‘green water’ syndrome: copper-induced hemolysis and subsequent acute renal failure as consequence of a religious ritual. Am J Med 1995; 98(3): 311–5
Dabrowska E, Jablonska-Kaszewska I, Ozieblowski A, et al. Acute haemolytic syndrome and liver failure as the first manifestations of Wilson’s disease. Med Sci Monit 2001; 7Suppl. 1: 246–51
Buchanan GR. Acute hemolytic anemia as a presenting manifestation of Wilson disease. J Pediatr 1975; 86(2): 245–7
Matsumura A, Hiraishi H, Terano A. Plasma exchange for hemolytic crisis in Wilson disease [letter]. Ann Intern Med 1999; 131(11): 866
Paglia DE, Valentine WN, Dahlgren JG. Effects of low-level lead exposure on pyrimidine 5′-nucleotidase and other erythrocyte enzymes: possible role of pyrimidine 5′-nucleotidase in the pathogenesis of lead-induced anemia. J Clin Invest 1975; 56(5): 1164–9
Hulten JO, Tran VT, Pettersson G. The control of haemolysis during transurethral resection of the prostate when water is used for irrigation: monitoring absorption by the ethanol method. BJU Int 2000; 86(9): 989–92
Knutsen OH, Jansson U. Hemolysis and pulmonary edema after a near-drowning accident in chlorated water [in Swedish]. Lakartidningen 1988; 85(52): 4646–7
Hubl W, Mostbeck B, Hartleb H, et al. Investigation of the pathogenesis of massive hemolysis in a case of Clostridium perfringens septicemia. Ann Hematol 1993; 67(3): 145–7
Sezerino UM, Zannin M, Coelho LK, et al. A clinical and epidemiological study of Loxosceles spider envenoming in Santa Catarina, Brazil. Trans R Soc Trop Med Hyg 1998; 92(5): 546–8
Murray LM, Seger DL. Hemolytic anemia following a presumptive brown recluse spider bite. J Toxicol Clin Toxicol 1994; 32(4): 451–6
Bey TA, Walter FG, Lober W, et al. Loxosceles arizonica bite associated with shock. Ann Emerg Med 1997; 30(5): 701–3
Leung LK, Davis R. Life-threatening hemolysis following a brown recluse spider bite. J Tenn Med Assoc 1995; 88(10): 396–7
Tambourgi DV, Morgan BP, de Andrade RM, et al. Loxosceles intermedia spider envenomation induces activation of an endogenous metalloproteinase, resulting in cleavage of glycophorins from the erythrocyte surface and facilitating complement-mediated lysis. Blood 2000; 95(2): 683–91
van Den Berg CW, de Andrade RM, Magnoli FC, et al. Loxosceles spider venom induces metalloproteinase mediated cleavage of MCP/CD46 and MHCI and induces protection against C-mediated lysis. Immunology 2002; 107(1): 102–10
Patel KD, Modur V, Zimmerman GA, et al. The necrotic venom of the brown recluse spider induces dysregulated endothelial cell-dependent neutrophil activation: differential induction of GM-CSF, IL-8, and E-selectin expression. J Clin Invest 1994; 94(2): 631–42
Campbell CH. Myotoxic paralysis and hemolytic anemia due to king brown snake bite. Aust N Z J Med 1984; 14(2): 169
Hung DZ, Wu ML, Deng JF, et al. Russell’s viper snakebite in Taiwan: differences from other Asian countries. Toxicon 2002; 40(9): 1291–8
Mukherje AK, Ghosal SK, Maity CR. Some biochemical properties of Russell’s viper (Daboia russelli) venom from Eastern India: correlation with clinico-pathological manifestation in Russell’s viper bite. Toxicon 2000; 38(2): 163–75
Phillips RE, Theakston RD, Warrell DA, et al. Paralysis, rhabdomyolysis and haemolysis caused by bites of Russell’s viper (Vipera russelli pulchella) in Sri Lanka: failure of Indian (Haffkine) antivenom. Q J Med 1988; 68(257): 691–715
Gillissen A, Theakston RD, Barth J, et al. Neurotoxicity, haemostatic disturbances and haemolytic anaemia after a bite by a Tunisian saw-scaled or carpet viper (Echis ‘pyramidum’-complex): failure of antivenom treatment. Toxicon 1994; 32(8): 937–44
Gibly RL, Walter FG, Nowlin SW, et al. Intravascular hemolysis associated with North American crotalid envenomation. J Toxicol Clin Toxicol 1998; 36(4): 337–43
Vetter RS, Visscher PK, Camazine S. Mass envenomations by honey bees and wasps. West J Med 1999; 170(4): 223–7
Munoz-Arizpe R, Valencia-Espinoza L, Velasquez-Jones L, et al. Africanized bee stings and pathogenesis of acute renal failure [letter]. Nephron 1992; 61(4): 478
Melvin JD, Watts RG. Severe hypophosphatemia: a rare cause of intravascular hemolysis. Am J Hematol 2002; 69(3): 223–4
Altuntas Y, Innice M, Basturk T, et al. Rhabdomyolysis and severe haemolytic anaemia, hepatic dysfunction and intestinal osteopathy due to hypophosphataemia in a patient after Billroth II gastrectomy. Eur J Gastroenterol Hepatol 2002; 14(5): 555–7
Kaiser U, Barth N. Haemolytic anaemia in a patient with anorexia nervosa. Acta Haematol 2001; 106(3): 133–5
Beutler E, Dern RJ, Alving AS. The hemolytic effect of primaquine: IV. The relationship of cell age to hemolysis. J Lab Clin Med 1954; 44(3): 439–42
Mason PJ. New insights into G6PD deficiency. Br J Haematol 1996; 94(4): 585–91
Beutler E. G6PD deficiency. Blood 1994; 84(11): 3613–36
Bulliamy T, Luzzatto L, Hirono A, et al. Hematologically important mutations: glucose-6-phosphate dehydrogenase. Blood Cells Mol Dis 1997; 23(2): 302–13
Nagel RL, Roth Jr EF. Malaria and red cell genetic defects. Blood 1989; 74(4): 1213–21
Luzzatto L, Usanga FA, Reddy S. Glucose-6-phosphate dehydrogenase deficient red cells: resistance to infection by malarial parasites. Science 1969; 164(881): 839–42
Steinberg MH, West MS, Gallagher D, et al. Effects of glucose-6-phosphate dehydrogenase deficiency upon sickle cell anemia. Blood 1988; 71(3): 748–52
Takizawa T, Huang IY, Ikuta T, et al. Human glucose-6-phosphate dehydrogenase: primary structure and cDNA cloning. Proc Natl Acad Sci USA 1986; 83(12): 4157–61
Beutler E, Vulliamy T, Luzzatto L. Hematologically important mutations: glucose-6-phosphate dehydrogenase. Blood Cells Mol Dis 1996; 22(1): 49–56
Beutler E, Vulliamy TJ. Hematologically important mutations: glucose-6-phosphate dehydrogenase. Blood Cells Mol Dis 2002; 28(2): 93–103
Kaplan M, Algur N, Hammerman C. Onset of jaundice in glucose-6-phosphate dehydrogenase-deficient neonates. Pediatrics 2001; 108(4): 956–9
Huang CS, Hung KL, Huang MJ, et al. Neonatal jaundice and molecular mutations in glucose-6-phosphate dehydrogenase deficient newborn infants. Am J Hematol 1996; 51(1): 19–25
Valaes T. Pathophysiology of spontaneous neonatal bilirubinemia associated with glucose-6-phosphate dehydrogenase deficiency. J Pediatr 1996; 128(6): 863–4
MacDonald MG. Hidden risks: early discharge and bilirubin toxicity due to glucose 6-phosphate dehydrogenase deficiency. Pediatrics 1995; 96 (4 Pt 1): 734–8
Fiorelli G, Martinez DM, Cappellini MD. Chronic non-spherocytic haemolytic disorders associated with glucose-6-phosphate dehydrogenase variants. Baillieres Best Pract Clin Haematol 2000; 13(1): 39–55
Chevion M, Navok T, Glaser G, et al. The chemistry of favism-inducing compounds: the properties of isouramil and divicine and their reaction with glutathione. Eur J Biochem 1982; 127(2): 405–9
Beutler E. Glucose-6-phosphate dehydrogenase deficiency. N Engl J Med 1991; 324(3): 169–74
Sklar GE. Hemolysis as a potential complication of acetaminophen overdose in a patient with glucose-6-phosphate dehydrogenase deficiency. Pharmacotherapy 2002; 22(5): 656–8
Wright RO, Perry HE, Woolf AD, et al. Hemolysis after acetaminophen overdose in a patient with glucose-6-phosphate dehydrogenase deficiency. J Toxicol Clin Toxicol 1996; 34(6): 731–4
Glucose-6-phosphate dehydrogenase deficiency. WHO Working Group. Bull World Health Organ 1989; 67(6): 601–11
Acknowledgements
The author is grateful to Ms Mary Lee for artwork and Dr John H. Matthews for the peripheral blood smears. No sources of funding were used to assist in the preparation of this review. The author has no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sivilotti, M.L.A. Oxidant Stress and Haemolysis of the Human Erythrocyte. Toxicol Rev 23, 169–188 (2004). https://doi.org/10.2165/00139709-200423030-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00139709-200423030-00004