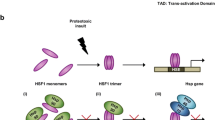
Abstract
A common feature of many neurodegenerative diseases, including Alzheimer’s and Parkinsons’s disease, the prion disorders, and the CAG repeat polyglutamine (polyQ) diseases, is the occurrence of protein aggregates within or outside of nerve cells. Molecular chaperones such as heat shock proteins (HSPs) have been proposed to play a critical role in preventing the accumulation of misfolded proteins that lead to the deposition of aggregates during pathology. This article focuses on the role of HSPs during polyQ pathologies, which include Huntington’s disease, spinal and bulbar muscular atrophy, dentatorubral and pallidoluysian atrophy, and several forms of spinocerebellar ataxia. Recently, unifying mechanisms that are involved during polyQ disease have emerged, such as abnormal transcription, impaired degradation systems, and interference of a polyQ expansion with neuronal survival and death-signaling pathways like the activation of caspases and kinases. This article reviews recent studies that point to the involvement of these mechanisms during polyQ pathology and discusses how HSPs can interfere with such processes by paying special attention to HSPs as modulators of survival and death-signaling pathways.
Similar content being viewed by others
References
Adachi H., Katsuno M., Minamiyama M., Sang C., Pagoulatos G., Angelidis C., et al. (2003) Heat shock protein 70 chaperone overexpression ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model by reducing nuclear-localized mutant androgen receptor protein. J. Neurosci. 23, 2203–2211.
Agarraberes F. A. and Dice J. F. (2001) A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J. Cell Sci. 114, 2491–2499.
Alberti S. and Hohfeld J. (2002) Molecular chaperones in the regulation of signal transduction. Ann. N. Y. Acad. Sci. 973, 3,4.
Alberti S., Demand J., Esser C., Emmerich N., Schild H., and Hohfeld J. (2002) Ubiquitylation of BAG-1 suggests a novel regulatory mechanism during the sorting of chaperone substrates to the proteasome. J. Biol. Chem. 277, 45920–45927.
Arrigo A.-P. (1998) Small stress proteins: chaperones that act as regulators of intracellular redox state and programmed cell death. Biol. Chem. 379, 19–26.
Ashkenazi A. and Dixit V. M. (1998) Death receptor: signaling and modulation. Science 281, 1305–1308.
Bailey C. K., Andriola I. F. M., Kampinga H. H., and Merry D. E. (2002) Molecular chaperones enhance the degradation of expanded polyglutamine repeat androgen receptor in a cellular model of spinal and bulbar muscular atrophy. Hum. Mol. Genet. 11, 515–523.
Beal M. F. (2000) Energetics in the pathogenesis of neurodegenerative diseases. Trends Neurosci. 23, 298–304.
Beere H. M., Wolf B. B., Cain K., Mosser D. D., Mahboubi A., Kuwana T., et al. (2000) Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2, 469–475.
Bence N. F., Roopal M., and Kopito R. R. (2001) Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292, 1552–1555.
Benn S. C., Perrelet D., Kato A. C., Scholz J., Decosterd I., Mannion R. J., et al. (2002) Hsp27 upregulation and phosphorylation is required for injured sensory and motor neurons survival. Neuron 36, 45–56.
Ben-Zvi A. P. and Goloubinoff P. (2001) Mechanisms of disaggregation and refolding of stable protein aggregates by molecular chaperones. J. Struct. Biol. 135, 84–93.
Bercovich B, Stancovski I., Mayer A., Blumenfeld N., Laszlo A., Schwartz A. L., and Ciechanover A. (1997) Ubiquitin-dependent degradation of certain protein substrates in vitro requires the molecular chaperone Hsc70. J. Biol. Chem. 272, 9002–9010.
Brazil D. P., Park J., and Hemmings B. A. (2002) PKB binding proteins. Getting on the Akt. Cell 111, 293–303.
Browne S. E., Ferrante R. J., and Beal M. F. (1999) Oxidative stress in Huntington’s disease. Brain Pathol. 9, 147–163.
Bruey J. M., Ducasse C., Bonniaud P., Ravagnan L., Susin S. A., Diaz-Latoud C., et al. (2000) Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell Biol. 2, 645–652.
Burnett B., Li F., and Pittman R. N. (2003) The polyglutamine neurodegenerative protein ataxin-3 binds polyubiquitylated proteins and has ubiquitin protease activity. Hum. Mol. Genet. 12, 3195–3205.
Busch A., Engemann S., Lurz R., Okazawa H., Lehrach H., and Wanker E. E. (2003) Mutant huntingtin promotes the fibrillogenesis of wild-type huntingtin: a potential mechanism for loss of huntingtin function in Huntington’s disease. J. Biol. Chem. 278, 41452–41461.
Carmichael J., Chatellier J., Woolfson A., Milstein C., Fersht A. R., and Rubinsztein D. C. (2001) Bacterial and yeast chaperones reduce both aggregate formation and cell death in mammalian cell models of Huntington’s disease. Proc. Natl. Acad. Sci. U. S. A. 97, 9701–9705.
Carmichael J., Sugars K. L., Bao Y. P., and Rubinsztein D. C. (2002) Glycogen synthase kinase-3β inhibitors prevent cellular polyglutamine toxicity caused by the Huntington’s disease mutation. J. Biol. Chem. 277, 33791–33798.
Cha J. H., Frey A. S., Alsdorf S. A., Kerner J. A., Kosinski C. M., Mangiarini L., et al. (1999) Altered neurotransmitter receptor expression in transgenic mouse models of Huntington’s disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354, 981–989.
Chai Y., Berke S. S., Cohen R. E., and Paulson H. L. (2003) Poly-ubiquitin binding by the polyglutamine disease protein ataxin-3 links its normal function to protein surveillance pathways. J. Biol. Chem. 279, 3605–3611.
Chai Y., Koppenhafer S. L., Bonini N. M., and Paulson H. L. (1999) Analysis of the role of heat shock protein (Hsp) molecular chaperones in polyglutamine disease. J. Neurosci. 19, 10338–10347.
Chan E. Y., Luthi-Carter R., Strand A., Solano S. M., Hanson S. A., DeJohn M. M., et al. (2002) Increased huntingtin protein length reduces the number of polyglutamine-induced gene expression changes in mouse models of Huntington’s disease. Hum. Mol. Gent. 11, 1939–1951.
Chan H. Y., Warrick J. M., Andriola I., Merry D., and Bonini N. M. (2002) Genetic modulation of polyglutamine toxicity by protein conjugation pathways in Drosophila. Hum. Mol. Genet. 11, 2895–2904.
Chan H. Y., Warrick J. M., Gray-Board G. L., Paulson H. L., and Bonini N. M. (2000) Mechanisms of chaperone suppression of polyglutamine disease: selectivity, synergy and modulation of protein solubility in Drosophila. Hum. Mol. Genet. 9, 2811–2820.
Charette S. J., Lavoie J. N., Lambert H., and Landry J. (2000) Inhibition of DAXX-mediated apoptosis by heat shock protein 27. Mol. Cell. Biol. 20, 7602–7612.
Chen H. K., Fernandez-Funez P., Acevedo S. F., Lam Y. C., Kaytor M. D., Fernandez M. H., et al. (2003) Interaction of Akt-phosphorylated ataxin-1 with 14-3-3 mediates neurodegeneration in spinocerebellar ataxia type 1. Cell 113, 457–68.
Chen S., Berthelier V., Hamilton J. B., O’Nuallain B., and Wetzel R. (2002) Amyloid-like features of polyglutamine aggregates and their assembly kinetics. Biochemistry 41, 7391–7399.
Chen S., Berthelier V., Yang W., and Wetzel R. (2001) Polyglutamine aggregation behaviour in vitro supports a recruitment mechanism of cytotoxicity. J. Mol. Biol. 311, 173–182.
Chernoff Y. O., Lindquist S. L., Ono B., Inge-Vechtomov S. G., and Liebman S. W. (1995) Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+]. Science 268, 880–884.
Clark J. I. and Muchowski P. J. (2000) Small heat-shock proteins and their potential role in human disease. Curr. Opin. Struct. Biol. 10, 52–59.
Clarke G., Collins R. A., Leavitt B. R., Andrews D. F., Hayden M. R., Lumsden C. J., and McInnes R. R. (2000) A one-hit model of cell death in inherited neuronal degenerations. Nature 406, 195–199.
Connell P., Ballinger C. A., Jiang J., Wu Y., Thompson L. J., Hohfeld J., Patterson C., et al. (2001) The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat. Cell. Biol. 3, 93–96.
Coux O., Tanaka K., and Goldberg A. L. (1996) Structure and function of the 20S and 26S proteasomes. Annu. Rev. Biochem. 65, 801–847.
Cowan K. J., Diamond M. I., and Welch W. J. (2003) Polyglutamine protein aggregation and toxicity are linked to cellular stress response. Hum. Mol. Genet. 12, 1377–1391.
Cuervo A. M. and Dice J. F. (2000) Age-related decline in chaperone-mediated autophagy. J. Biol. Chem. 275, 31505–31513.
Cummings C. J. and Zoghbi H. Y. (2000a) Fourteen and counting: unraveling trinucleotide repeat diseases. Hum Mol Genet. 9, 909–916.
Cummings C. J. and Zoghbi H. Y. (2000b) Trinucleotide repeats: mechanisms and pathophysiology. Annu. Rev. Genomics Hum. Genet. 1, 281–328.
Cummings C. J., Mancini M. A., Antalffy B., DeFranco D. B., Orr H. T., and Zoghbi H. Y. (1998) Chaperone suppression of aggregation and altered subcellular proteasome localization imply protein misfolding in SCA1. Nat. Genet. 19, 148–154.
Cummings C. J., Reinstein E., Sun Y., Antalffy B., Jiang Y., Ciechanover A., et al. (1999) Mutation of the E6-AP ubiquitin ligase reduces nuclear inclusion frequency while accelerating polyglutamine-induced pathology in SCA1 mice. Neuron 24, 879–892.
Cummings C. J., Sun Y., Opal P., Antalffy B., Mestril R., Orr H. T., et al. (2001) Over-expression of inducible HSP70 chaperone suppresses neuropathology and improves motor function in SCA1 mice. Hum. Mol. Genet. 10, 1511–1518.
Deckel A. W. (2001) Nitric oxide and nitric oxide synthase in Huntington’s disease. J. Neurosci. Res. 64, 99–107.
Demand J., Alberti S., Patterson C., and Hahfeld J. (2001) Cooperation of a ubiquitin domain protein and an E3 ubiquitin ligase during chaperone/proteasome coupling. Curr. Biol. 11, 1569–1577.
DiFiglia M., Sapp E., Chase K. O., Davies S. W., Bates G. P., Vonsattel J. P., and Aronin N. (1997) Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277, 1990–1993.
Donaldson K. M., Li W., Ching K. A., Batalov S., Tsai C. C., and Joazeiro C. A. (2003) Ubiquitin-mediated sequestration of normal cellular proteins into polyglutamine aggregates. Proc. Natl. Acad. Sci. USA 100(15), 8892–8897.
Doss-Pepe E. W., Stenroos E. S., Johnson W. G., and Madura K. (2003) Ataxin-3 interactions with rad23 and valosin-containing protein and its associations with ubiquitin chains and the proteasome are consistent with a role in ubiquitin-mediated proteolysis. Mol. Cell. Biol. 23(18), 6469–6483.
Dunah A. W., Jeong H., Griffin A., Kim Y. M., Standaert D. G., Hersch S. M., et al., (2002) Sp1 and TAFII130 transcriptional activity disrupted in early Huntington’s disease. Science 296, 2238–2243.
Eaton P., Fuller W., and Shattock M. J. (2002) S-thiolation of HSP27 regulates its multimeric aggregate size independently of phosphorylation. J. Biol. Chem. 277, 21189–21196.
Emamian E. S., Kaytor M. D., Duvick L. A., Zu T., Tousey S. K., Zoghbi H. Y., et al. (2003) Serine 776 of ataxin-1 is critical for polyglutamine-induced disease in SCA1 transgenic mice. Neuron 38, 375–387.
Evert B.O., Vogt I. R., Vieira-Saecker A. M., Ozimek L., de Vos R. A., Brunt E. R., et al. (2003) Gene expression profiling in ataxin-3 expressing cell lines reveals distinct effects of normal and mutant ataxin-3. J. Neuropathol. Exp. Neurol. 62, 1006–1018.
Fernandez-Funez P., Nino-Rosales M. L., de Gouyon B., She W. C., Luchak J. M., Martinez P., et al. (2000) Identification of genes that modify ataxin-1-induced neurodegeneration. Nature 408, 101–106.
Ferrante R. J., Andreassen O. A., Dedeglu A., Ferrante K. L., Jenkins B. G., Hersch S. M., and Beal M. F. (2002) Therurapeutic effects of coenzyme Q10 and Remacemide in transgenic mouse models of Huntington’s disease. J. Neurosci. 22, 1592–1599.
Fleury C., Mignotte B., and Vayssiere J. L. (2002) Mitochondrial oxygen species in cell death signaling. Biochimie 84, 131–141.
Gabai V. L., Meriin A. B., Yaglom J. A., Wei J. Y., Mosser D. D., and Sherman M. Y., (2000a) Suppression of stress kinase JNK is involved in HSP72-mediated protection of myogenic cells from transient energy deprivation. HSP72 alleviates the stress-induced inhibition of JNK dephosphorylation. J. Biol. Chem. 275, 38088–38094.
Gabai V. L., Yaglom J. A., Volloch V., Meriin A. B., Force T., Koutroumanis M., et al. (2000b) Hsp70-mediated suppression of c-Jun N-terminal kinase is implicated in development of tolerance to caspase-independent cell death. Mol. Cell. Biol. 20, 6826–6836.
Garcia-Mata R., Bebok Z., Sorscher E. J., and Sztul E. S. (1999) Characterization and dynamics of aggresome formation by a cytosolic GFP-chimera. J. Cell. Biol. 146, 1239–1254.
Garrido C., Bruey J. M., Fromentin A., Hammann A., Arrigo A. P., and Solary E. (1999) HSP27 inhibits cytochrome c-dependent activation of procaspase-9. FASEB J. 13, 2061–2070.
Gervais F. G., Singaraja R., Xanthoudakis S., Gutekunst C. A., Leavitt B. R., Metzler M., et al. (2002) Recruitment and activation of caspase-8 by the huntingtin-interacting protein Hip-1 and a novel partner Hippi. Nat. Cell. Biol. 4, 95–105.
Giuliano P., De Cristofaro T., Affaitati A., Pizzulo G. M., Feliciello A., Criscuolo C., et al. (2003) DNA damage induced by polyglutamine-expanded proteins. Hum. Mol. Genet. 12, 2301–2309.
Green D. R. (1998) Apoptotic pathways: the roads to ruin. Cell 94, 695–698.
Green G. R. (1999) Harm’s way: polyglutamine repeats and the activation of an apoptotic pathway. Neuron 22, 416–417.
Guidetti P., Charles V., Chen E. Y., Reddy P. H., Kordower J. H., Whetsell W. O., Jr., et al. (2001) Early degenerative changes in transgenic mice expressing mutant huntingtin involve dendritic abnormalities but no impairment of mitochondrial energy production. Exp. Neurol. 169, 340–350.
Gunawardena S., Her L. S., Brusch R. G., Laymon R. A., Niesman I. R., Gordesky-Gold B., et al. (2003) Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron 40, 25–40.
Gurbuxani S., Schmitt E., Cande C., Parcellier A., Hammann A., Daugas E., et al. (2003) Heat shock protein 70 binding inhibits the nuclear import of apoptosis-inducing factor. Oncogene 22, 6669–6678.
Gusella J. F. and McDonald M. E. (2000) Molecular genetics: unmasking polyglutamine triggers in neurodegenerative disease. Nat. Rev. Neurosci. 1, 109–115.
Gutekunst C. A., Li S. H., Yi H., Ferrante R. J., Li X. J., and Hersch S. M. (1998) The cellular and subcellular localisation of huntingtin-associated protein 1 (HAP-1): comparison with huntingtin in rat and human. J. Neurosci. 18, 7674–7686.
Hansson O., Nylandsted J., Castilho R. F., Leist M., Jaattela M., and Brundin P. (2003) Overexpress on of heat shock protein 70 in R6/2 Huntington’s disease mice has only modest effects on disease progression. Brain Res. 970, 47–57.
Harper S. J. and LoGrasso P. (2001) Signalling for survival and death in neurones: the role of stress-activated kinases, JNK and p38. Cell Signal. 13, 299–310.
Hartl F. U. (1996) Molecular chaperones in cellular protein folding. Nature 381, 571–579.
Hartl F. U. and Hayer-Hartl M. (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295, 1852–1858.
Higashiyama H., Hirose F., Yamaguchi M., Inoue Y. H., Fujikake N., Matsukage A., and Kakizuka A. (2002) Identification of ter94, Drosophila VCP, as a modulator of polyglutamine-induced neurodegeneration. Cell Death Differ. 9, 264–273.
Hochstrasser M. (1996) Ubiquitin-dependent protein degradation. Annu. Rev. Genet. 30, 405–439.
Holbert S., Denghien I., Kiechle T., Rosenblatt A., Wellington C., et al. (2001)
The Gln-Ala repeat transcriptional activator CA 150 interacts with huntingtin: neuropathologic and genetic evidence for a role in Huntington’s disease pathogenesis. Proc. Natl. Acad. Sci. USA 98, 1811–1816.
Hsu A. L., Murphy C. T., and Kenyon C. (2003) Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300, 1142–1145.
Humbert S., Bryson E. A., Cordelieres F. P., Connors N. C., Datta S. R., Finkbeiner S., et al. (2002) The IGF-1/Akt pathway is neuroprotective in Huntington’s disease and involves Huntingtin phosphorylation by Akt. Dev. Cell 2, 831–837.
Ishihara K., Yamagishi N., Saito Y., Adachi H., Kobayashi Y., Sobue G., et al. (2003) Hsp105α suppresses the aggregation of truncated androgen receptor with expanded CAG repeats and cell toxicity. J. Biol. Chem. 278, 25143–25150.
Jackson G. R., Salecker I., Dong X., Yao X., Arnheim N., Faber P. W., et al. (1998) Polyglutamine-expanded human huntingtin transgenes induce degeneration of Drosophila photoreceptor neurons. Neuron 21, 633–642.
Jana N. R., Tanaka M., Wang G. H., and Nukina N. (2000) Polyglutamine length dependent interaction of Hsp40 and HSP70 family chaperones with truncated N-terminal huntingtin: their suppression of aggregation and cellular toxicity Hum. Mol. Genet. 9, 2009–2018.
Jana N. R., Zemskov E. A., Wang G. H., and Nukina N. (2001) Altered proteasomal function due to the expression of polyglutamine-expanded truncated N-terminal huntingtin induces apoptosis by caspase activation through mitochondrial cytochrome crelease. Hum. Mol. Genet. 10, 1049–1059.
Johnston J. A., Ward C. L., and Kopito R. R. (1998) Aggresomes: a cellular response to misfolded proteins. J. Cell. Biol. 143, 1883–1898.
Jolly C. and Morimoto R. I. (2000) Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J. Natl. Cancer Inst. 92, 1564–1572.
Jones L. (1999) The localisation and interactions of huntingtin. Philos. Trans. R Soc. Lond. B Biol. Sci. 354, 1021–1027.
Kaplan D. R. and Miller F. D. (2000) Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 10, 381–391.
Kato H., Liu Y., Kogure K., and Kato K. (1994) Induction of 27 kDa heat shock protein following cerebral ischemia in a rat model of ischemic tolerance. Brain Res. 634, 235–244.
Kaytor M. D., Duvick L. A., Skinner P. J., Koob M. D., Ranum L. P., and Orr H. T. (1999) Nuclear localization of the spinocerebellar ataxia type 7 protein, ataxin-7. Hum. Mol. Genet. 8, 1657–1664.
Kazantsev A., Preisinger E., Dranovsky A., Goldgaber D., and Housman D. (1999) Insoluble detergent-resistant aggregates form between pathological and nonpathological lengths of polyglutamine in mammalian cells. Proc. Natl. Acad. Sci. USA 96, 11404–11409.
Kazemi-Esfarjani P. and Benzer S. (2000) Genetic suppression of polyglutamine toxicity in Drosophila. Science 287, 1837–1840.
Kegel K. B., Kim M., Sapp E., McIntyre C., Castano J. G., Aronin N., and DiFiglia M. (2000) Huntingtin expression stimulates endosomal-lysosomal activity, endosome tubulation, and autophagy. J Neurosci. 20, 7268–7278.
Kiechle T., Dedeoglu A., Kubilus J., Kowall N. W., Beal M.F., Friedlander R., et al. (2002) Cytochrome c and caspase-9 expression in Huntington’s disease. Neuromol. Med. 1, 183–195.
Kim M., Lee H. S., LaForet G., McIntyre C., Martin E. J., Chang P., et al. (1999) Mutant huntingtin expression in clonal striatal cells: dissociation of inclusion formation and neuronal survival by caspase inhibition. J. Neurosci. 19, 964–973.
Kim S., Nollen E. A, Kitagawa K., Bindokas V. P., and Morimoto R. I. (2002) Polyglutamine protein aggregates are dynamic. Nat. Cell. Biol. 4, 826–831.
Kisselev A. F., Akopian T. N., Woo K. M., and Goldberg A. L. (1999) The sizes of peptides generated from protein by mammalian 26 and 20S proeasomes. Implications for understanding the degradative mechanisms and antigen presentation. J. Biol. Chem. 274, 3363–3371.
Kita H., Carmichael J., Swartz J., Muro S., Wyttenbach A., Matsubara K., et al. (2002) Modulation of polyglutamine-induced cell death by genes identified by expression profiling. Hum. Mol. Genet. 11, 2279–2287.
Kobayashi Y., Kume A., Li M., Doyu M., Hata M., Ohtsuka K., and Sobue G. (2000) Chaperones Hsp70 and Hsp40 suppress aggregate formation and apoptosis in cultured neuronal cells expressing truncated androgen receptor protein with expanded polyglutamine tract. J. Biol. Chem. 275, 8772–8778.
Konishi H., Matsuzaki H., Tanaka M., Takemura Y., Kuroda S., Ono Y., and Kikkawa U. (1997) Activation of protein kinase B (Akt/RAC-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS Lett. 410, 493–498.
Kopito R. R. (2000) Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 10, 524–530.
Kouroku Y., Fujita E., Jimbo A., Kikuchi T., Yamagata T., Momoi M. Y., et al. (2002) Polyglutamine aggregates stimulate ER stress signals and caspase-12 activation. Hum. Mol. Genet. 11, 1505–1515.
Krobitsch S. and Lindquist S. (2000) Aggregation of huntingtin in yeast varies with the length of the polyglutamine expansion and the expression of chaperone proteins. Proc. Natl. Acad. Sci. USA 97, 1589–1594.
La-Fevre-Bernt M. A. and Ellerby L. M. (2003) Kennedy’s disease. Phosphorylation of the polyglutamine-expanded form of androgen receptor regulates its cleavage by caspase-3 and enhances cell death. J. Biol. Chem. 278, 34918–34924.
Lemon B. and Tjian R. (2000) Orchestrated response: a symphony of transcription factors for gene control. Genes Dev. 14, 2551–2569.
Li H., Yu Z. Z., Shelbourne P., and Li X. J. (2001) Huntingtin aggregate associated axonal degeneration is an early pathological event in Huntington’s disease. J. Neurosci. 21, 8473–8481.
Li H., Zhu H., Xu C. J., and Yuan J. (1998) Cleavage of BID by caspase-8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94, 491–501.
Li L. Y., Luo X., and Wang X. (2001) Endonuclease G is an apoptotic Dnase when released from mitochondria. Nature 412, 95–99.
Li S. H., Lam S., Cheng A. L, and Li X. J. (2000) Intranuclear huntingtin increases the expression of caspase-1 and induces apoptosis. Hum. Mol. Genet. 9, 2859–2867.
Lieberman A. P., Harmison G., Strand A. D., Olson J. M., and Fischbeck K. H. (2002) Altered transcriptional regulation in cells expressing the expanded polyglutamine androgen receptor. Hum. Mol. Genet. 11, 1967–1976.
Lin X., Antalffy B., Kang D., Orr H. T., and Zoghbi H. Y. (2000) Polyglutamine expansion down-regulates specific neuronal genes before pathologic changes in SCA1. Nat. Neurosci. 3, 157–163.
Liu Y. F. (1998) Expression of polyglutamine-expanded Huntingtin activates the SEK1-JNK pathway and induces apoptosis in a hippocampal neuronal cell line. J. Biol. Chem. 273(44), 28873–28877.
Liu Y. F., Dorow D., and Marshall J. (2000) Activation of MLK2-mediated signaling cascades by polyglutamine-expanded huntingtin. J. Biol. Chem. 275, 19035–19040.
Lockshin R. A. and Zakeri Z. (2002) Caspase-independent cell deaths. Curr. Opin. Cell. Biol. 14, 727–733.
Lonze B. E. and Ginty D. D. (2002) Function and regulation of CREB family transcription factors in the nervous system. Neuron 35, 605–623.
Ludwig S., Engel K., Hoffmeyer A., Sithanandam G., Neufeld B., Palm D., et al. (1996) 3pK, a novel mitogenactivated protein (MAP) kinase-activated protein kinase, is targeted by three MAP-kinase pathways. Mol. Cell. Biol. 16, 6687–6697.
Luthi-Carter R., Hanson S. A., Strand A. D., Bergstrom D. A., Chun W., Peters N. L., et al. (2002a) Dysregulation of gene expression in the R6/2 model of polyglutamine disease: parallel changes in muscle and brain. Hum. Mol. Genet. 11, 1911–1926.
Luthi-Carter R., Strand A. D., Hanson S. A., Kooperberg C., Schilling G., La Spada A. R., et al. (2002b) Polyglutamine and transcription: gene expression changes shared by DRPLA and Huntington’s disease mouse models reveal context-independent effects. Hum. Mol. Genet. 11, 1927–1937.
Luthi-Carter R., Strand A., Peters N. L., Solano S. M., Hollingsworth Z. R., Menon A. S., et al. (2000) Decreased expression of striatal signaling genes in a mouse model of Huntington’s disease. Hum. Mol. Genet. 9, 1259–1271.
Martinou J. C. and Green D. R. (2001) Breaking the mitochondrial barrier. Nat. Rev. Cell. Biol. 2, 63–67.
Mattson M. P., Chan S. L., and Duan W. (2002) Modification of brain aging and neurodegenerative disorders by genes, diet and behavior. Physiol. Rev. 82, 637–672.
McCampbell A., Taylor J. P., Taye A. A., Robitschek J., Li M., Walcott J., et al. (2000) CREB-binding protein sequestration by expanded polyglutamine. Hum. Mol. Genet. 9, 2197–2202.
Mearow K. M., Dodge M. E., Rahimtula M., and Yegappan C. (2002) Stress-mediated signaling in PC12 cells—the role of the small heat shock protein, Hsp27, and Akt in protecting cells from heat stress and nerve growth factor withdrawal. J. Neurochem. 83, 452–462.
Mehlen P., Hickey E., Weber L. A., and Arrigo A. P. (1997) Large unphosphorylated aggregates as the active form of hsp27 which controls intracellular reactive oxygen species and glutathione levels and generates a protection against TNFα in NIH-3T3-ras cells. Biochem. Biophys. Res. Commun. 241, 187–192.
Merienne K., Helmlinger D., Perkin G. R., Devys D., and Trottier Y. (2003) Polyglutamine expansion induces a protein-damaging stress connecting heat shock protein 70 to the JNK pathway. J. Biol Chem. 278, 16957–16967.
Meriin A. B., Yaglom J. A., Gabai V. L., Zon L., Ganiatsas S., Mosser D. D., et al. (1999) Protein-damaging stresses activate c-Jun N-terminal kinase via inhibition of its dephosphorylation: a novel pathway controlled by HSP72. Mol. Cell. Biol. 19, 2547–2555.
Mitsui K., Nakayama H., Akagi T., Nekooki M., Ohyawa K., Takio K., et al. (2002) Purification of polyglutamine aggregates and identification of elongation factor-1a and heat shock protein 84 as aggregate-interacting proteins. J. Neurosci. 22, 9267–9277.
Miyashita T., Ohtsuka Y., Okamura-Oho Y., Shikama Y., and Yamada M. (2001) Extended polyglutamine selectively interacts with caspase-8 and -10 in nuclear aggregates. Cell Death Differ. 8, 377–386.
Morimoto R. I. (2002) Dynamic remodeling of transcription complexes by molecular chaperones. Cell 110, 281–284.
Morimoto R. I., Kline M. P., Bimston D. N., and Cotto J. J. (1997) The heat-shock response: regulation and function of heat-shock proteins and molecular chaperones. Essays Biochem. 32, 17–29.
Mosser D. D., Caron A. W., Bourget L., Meriin A. B., Sherman M. Y., Morimoto R. I., and Massie B. (2000) The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol. Cell. Biol. 20, 7146–7159.
Moulder K. L., Onodera O. Burke J. R., Strittmatter W. J., and Johnson, E. M., Jr. (1999) Generation of neuronal intranuclear inclusions by polyglutamine-GFP: analysis of inclusion clearance and toxicity as a function of polyglutamine length. J. Neurosci. 19, 705–715.
Muchowski P. J., Ning K., D’Souza-Schorey C., and Fields S. (2002) Requirement of an intact microtubule cytoskeleton for aggregation and inclusion body formation by a mutant huntingtin fragment. Proc. Natl. Acad. Sci. USA 99, 727–732.
Muchowski P.J., Schaffar G., Sittler A., Wanker E. E., Hayer-Hartl M. K., and Hartl F. U. (2000) Hsp70 and hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc. Natl. Acad. Sci. USA 97, 7841–7846.
Muzio M., Chinnaiyan A. M., Kischkel F. C., O’Rourke K., Shevchenko A., Ni J., et al. (1996) FLICE, a novel FADD-homologous ICE/CED-3 like protease, is recruited to the CD95 (Fas/Apo-1) death-inducing signaling complex. Cell 85, 817–827.
Narain Y., Wyttenbach A., Rankin J., Furlong R. A., and Rubinzstein D. C. (1999) A molecular investigation of true dominance in Huntington’s disease. J. Med. Genet. 36, 739–746.
Nishitoh H., Matsuzawa A., Tobiume K., Saegusa K., Takeda K., Inoue K., et al. (2002) ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 16, 1345–1355.
Nucifora F. C., Jr., Sasaki M., Peters M.F., Huang H., Cooper J. K., Yamada M., et al. (2001) Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science 291, 2423–2428.
Opal P. and Zoghbi H. Y. (2002) The role of chaperones in polyglutamine disease. Trends Mol. Med. 8, 232–236.
Pandey P., Farber R., Nakazawa A., Kumar S., Bharti A., Nalin C., et al. (2000a) Hsp27 functions as a negative regulator of cytochrome c-dependent activation of procaspase-3. Oncogene 19, 1975–1981.
Pandey P., Saleh A., Nakazawa A., Kumar S., Srinivasula S. M., Kumar V., et al. (2000b) Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 19, 4310–4322.
Parcellier A., Schmitt E., Gurbuxani S., Seigneurin-Berny D., Pance A., Chantome A., et al. (2003) HSP27 is a ubiquitin-binding protein involved in IκBα proteasomal degradation. Mol. Cell. Biol. 23, 5790–5802.
Park H. S., Cho S. G., Kim C. K., Hwang H. S., Noh K. T., Kim M. S., et al. (2002) Heat shock protein hsp72 is a negativeregulator of apoptosis signal-regulating kinase 1. Mol. Cell. Biol. 22(22), 7721–7730.
Park H. S., Lee J. S., Huh S. H., Seo J. S., and Choi E. J. (2001) Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J. 20, 446–456.
Patino M. M., Liu J. J., Glover J. R., and Lindquist S. (1996) Support for the prion hypothesis for inheritance of a phenotypic trait in yeast. Science 273, 622–626.
Paulson H. L., Bonini N. M., and Roth K. A. (2000) Polyglutamine disease and neuronal cell death. Proc. Natl. Acad. Sci. USA 97, 12957,12958.
Perez M. K., Paulson H. L., Pendse S. J., Saionz S. J., Bonini N. M., and Pittman R. N. (1998) Recruitment and the role of nuclear localisation in polyglutamine-mediated aggregation. J. Cell Biol. 143, 1457–1470.
Perutz M. F. (1999) Glutamine repeats and neurodegenerative diseases: molecular aspects. Trends Biochem. Sci. 24, 58–63.
Perutz M. F. and Windle A. H. (2001) Cause of neural death in neurodegenerative diseases attributable to expansions of glutamine repeats. Nature 412, 143–144.
Perutz M. F., Johnson T., Suzuki M., and Finch J. T. (1994) Glutamine repeats as polar zippers: their possible role in inherited neurodegenerative diseases. Proc. Natl. Acad. Sci. USA 91, 5355–5358.
Perutz M. F., Pope B. J., Owen D., Wanker E. E., and Scherzinger E. (2002) Aggregation of proteins with expanded glutamine and alanine repeats of the glutamine-rich and asparagine-rich domains of Sup35 and of the amyloid beta-peptide of amyloid plaques. Proc. Natl. Acad. Sci. USA 99, 5596–5600.
Portera-Cailliau C., Hedreen J. C., Price D. L., and Koliatsos V. E. (1995) Evidence for apoptotic cell death in Huntington disease and excitotoxic animal models. J. Neurosci. 15, 3775–3787.
Preisinger E., Jordan B. M., Kazantsev A., and Housman D. (1999) Evidence for a recruitment and sequestration mechanism in Huntington’s disease. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 354, 1029–1034.
Rane M. J., Pan Y., Singh S., Powell D. W., Wu R., Cummins T., et al. (2003) Heat shock protein 27 controls apoptosis by regulating Akt activation. J. Biol. Chem. 278, 27828–27835.
Ravagnan L., Gurbuxani S., Susin S. A., Maisse C., Daugas E., Zamzami N., et al. (2001) Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat. Cell. Biol. 3, 839–843.
Ravikumar B., Duden R., and Rubinsztein D. C. (2002) Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum. Mol. Genet. 11, 1107–1117.
Ravikumar B. Stewart A., Kita H., Kato K., Duden R., and Rubinsztein D. C. (2003) Raised intracellular glucose concentrations reduce aggregation and cell death caused by mutant huntingtin exon 1 by decreasing mTOR phosphorylation and inducing autophagy. Hum. Mol. Genet. 12, 985–994.
Reddy P. H., Williams M., Charles V., Garret L., Pike-Buchanan L., Whetsell W. O., et al. (1998) Behavioural abnormalities and selective neuronal loss in HD transgenic mice expressing mutated full-length HD cDNA. Nat. Genet. 20, 198–202.
Reggiori F. and Klionsky D. J. (2002) Autophagy in the eukaryotic cell. Eukaryot. Cell 1, 11–21.
Rigamonti D., Bauer J. H., De-Fraja C., Conti L., Sipione S., Sciorati C., et al. (2000) Wild-type huntingtin protects from apoptosis upstream of caspase-3. J Neurosci. 20, 3705–3713.
Rochet J. C. and Lansbury P. T., Jr. (2000) Amyloid fibrillogenesis: themes and variations. Curr. Opin. Struct. Biol. 10, 60–68.
Rock K. L., Gramm C., Rothstein L., Clark K., Stein R., Dick L., et al. (1994) Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78, 761–771.
Rogalla T., Ehrnsperger M., Preville X., Kotlyarov A., Lutsch G., Ducasse C., et al. (1999) Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J. Biol. Chem. 274, 18947–18956.
Ross C. A. (1995) When more is less: pathogenesis of glutamine repeat neurodegenerative diseases. Neuron 15, 493–496.
Ross C. A. (2002) Polyglutamine pathogenesis: emergence of unifying mechanisms for Huntington’s disease and related disorders. Neuron 35, 819–822.
Ross C. A., Poirier M. A., Wanker E. E., and Amzel M. (2003) Polyglutamine fibrillogenesis: the pathway unfolds. Proc. Natl. Acad. Sci. USA 100, 1–3.
Rubinsztein D. C. and Hayden M. R. (1998) Analysis of Triplet Disorders. BiosScientific Publishers, Oxford, UK, p. 327.
Sakahira H., Breuer P., Hayer-Hartl M. K., and Hartl F. U. (2002) Molecular chaperones as modulators of polyglutamine protein aggregation and toxicity. Proc. Natl. Acad. Sci. USA 99(Suppl. 4), 16412–16418.
Saleh A., Srinivasula S. M., Balkir L., Robbins P. D., and Alnemri E. S. (2000) Negative regulation of the Apaf-1 apoptosome by Hsp70. Natl. Cell Biol. 2, 476–483.
Sanchez I., Xu C. J., Juo P., Kakizaka A., Blenis J., and Yuan J. (1999) Caspase-8 is required for cell death induced by expanded polyglutamine repeats. Neuron 22, 623–633.
Sapp E., Schwarz C., Chase K., Bhide P. G., Young A. B., et al. (1997) Huntingtin localization in brains of normal and Huntington’s disease patients. Ann. Neurol. 42, 604–612.
Sato S., Fujita N., and Tsuruo T. (2000) Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. USA 97, 10832–10837.
Satyal S. H., Schmidt E., Kitagawa K., Sondheimer N., Lindquist S., Kramer J. M., and Morimoto R. I. (2000) Polyglutamine aggregates alter protein folding homeostasis in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 97, 5750–5755.
Saudou F., Finkbeiner S., Devys D., and Greenberg M. E. (1998) Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell 95, 55–66.
Scherzinger E., Lurz R., Turmaine M., Mangiarini L., Hollenbach B., et al. (1997)
Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell 90, 549–558.
Scherzinger E., Sittler A., Schweiger K., Heiser V., Lurz R., Hasenbank R., et al. (1999) Self-assembly of polyglutamine-containing huntingtin fragments into amyloid-like fibrils: implications for Huntington’s disease pathology. Proc. Natl. Acad. Sci. USA 96, 4604–4609.
Sherman M. Y. and Goldberg A. L. (2001) Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron 29, 15–32.
Shimohata T., Nakajima T., Yamada M., Uchida C., Onodera O., Naruse S., et al. (2000) Expanded polyglutamine stretches interact with TAFII130, interfering with CREB-dependent transcription. Nat. Genet. 26, 29–36.
Sipione S., Rigamonti D., Valenza M., Zuccato C., Conti L., Pritchard J., et al. (2002) Early transcriptional profiles in huntingtin-inducible striatal cells by microarray analyses. Hum. Mol. Genet. 11, 1953–1965.
Sittler A., Lurz R., Lueder, G., Priller J., Lehrach H., Hayer-Hartl M. K., et al. (2000) Geldanamycin activates a heat shock response and inhibits huntingtin aggregation in a cell culture model of Huntington’s disease. Hum. Mol. Genet. 10, 1307–1315.
Skinner P. J. Koshy B. T., Cummings C. J., Klement I. A., Helin K., Servadio A., et al. (1997) Ataxin-1 with an expanded glutamine tract alters nuclear matrix-associated structures. Nature 389, 971–978.
Song Y., Cardounel A. J., Zweier J. L., and Xia Y. (2002) Inhibition of superoxide generation from neuronal nitric oxide synthase by heat shock protein 90: implications in NOS regulation. Biochemistry 41, 10616–10622.
Soti C. and Csermely P. (2002) Chaperones and aging: role in neurodegeneration and in other civilizational diseases. Neurochem. Int. 41, 383–389.
Steffan J. S., Bodai L., Pallos J., Poelman M., McCampbell A., et al. (2001) Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 413, 739–743.
Steffan J. S., Kazantsev A., Spasic-Boskovic O., Greenwald M., Zhu Y. Z., Gohler H., et al. (2000) The Huntington’s disease protein interacts with p53 and CREB-binding protein and represses transcription. Proc. Natl. Acad. Sci. USA 97, 6763–6768.
Stenoien D. L., Cummings C. J., Adams H. P., Mancini M. G., Patel K., DeMartino G. N., et al. (1999) Polyglutamine-expanded androgen receptors form aggregates that sequester heat shock proteins, proteasome components and SRC-1, and are suppressed by the HDJ-2 chaperone. Hum. Mol. Genet. 8, 731–741.
Stenoien D. L., Mielke M., and Mancini M. A. (2002) Intranuclear ataxin1 inclusions contain both fast- and slow-exchanging components. Nat. Cell. Biol. 4, 806–810.
Stokoe D., Engel K., Campbell D. G., Cohen P., and Gaestel M. (1992) Identification of MAPAKAP kinase 2 as a major enzyme responsible for the phosphorylation of small mammalian heat shock proteins. FEBS Lett. 313, 307–313.
Suhr S. T., Senut M. C., Whitelegge J. P., Faull K. F., Cuizon D. B., and Gage F. H. (2001) Identities of sequestered proteins in aggregates from cells with induced polyglutamine expression. J. Cell. Biol. 153, 283–294.
Susin S. A., Lorenzo N., Zamzami I., Marzo B. E., Snow G. M., Brothers J., et al. (1999) Molecular characterisation of mitochondrial apoptosis-inducing factor. Nature 397, 441–446.
Szebenyi G., Morfini G. A., Babcock A., Gould M., Selkoe K., Stenoien D. L., et al. (2003) Neuropathogenic forms of huntingtin and androgen receptor inhibit fast axonal transport. Neuron 40, 41–52.
Tabrizi S. J., Workman J., Hart P. E., Mangiarini L., Mahal A., Bates G., et al. (2000) Mitochondrial dysfunction and free radical damage in the Huntington’s R6/2 transgenic mouse. Ann. Neurol. 47, 80–86.
Tanaka M., Machida Y., Nishikawa Y., Akagi T., Hashikawa T., Fujisawa T., and Nukina N. J. (2003) Expansion of polyglutamine induces the formation of quasiaggregate in the early stage of protein fibrillization. J. Biol. Chem. 278, 34717–34724.
Taylor J. P., Tanaka F., Robitschek J., Sandoval C. M., Taye A., Markovic-Plese S., and Fischbeck K. H. (2003) Aggresomes protect cells by enhancing the degradation of toxic polyglutamine-containing protein. Hum. Mol. Genet. 12, 749–757.
Tobiume K., Matsuzawa A., Takahashi T., Nishitoh H., Morita K., Takeda K., et al. (2001) ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2, 222–228.
Toyoshima I., Sugawara M., Kato K., Wada C., Shimohata T., Koide R., et al. (2002) Time course of polyglutamine aggregate body formation and cell death: enhanced growth in the nucleus and an interval for cell death. J. Neurosci. Res. 68, 442–448.
Tsai Y. C., Fishman P. S., Thakor N. V., and Olyer G. A. (2003) Parkin facilitates the elimination of expanded polyglutamine proteins and leads to preservation of proteasome function. J. Biol. Chem. 278, 22044–22055.
Turmaine M., Raza A., Mahal A., Mangiarini L., Bates G. P., and Davies S. W. (2000) Nonapoptotic neurodegeneration in a transgenic mouse model of Huntington’s disease. Proc. Natl. Acad. Sci. USA 97, 8093–8097.
Verhoef L. G., Lindsten K., Masucci M. G., and Dantuma N. P. (2002) Aggregate formation inhibits proteasomal degradation of polyglutamine proteins. Hum. Mol. Genet. 11, 2689–2700.
Vleminckx V., Van Damme P., Goffin K., Delye H., Van Den Bosch L., and Robberecht W. (2002) Upregulation of HSP27 in a transgenic model of ALS. J. Neuropathol. Exp. Neurol. 61, 968–974.
Waelter S., Boeddrich A., Lurz R., Scherzinger E., Lueder G., Lehrach H., and Wanker E. E. (2001) Accumulation of mutant huntingtin fragments in aggresome-like inclusion bodies as a result of insufficient degradation. Mol. Biol. Cell. 12, 1393–1407.
Wagstaff M. J., Collaco-Moraes Y., Smith J., de Belleroche J. S., Coffin R. S., and Latchman D. S. (1999) Protection of neuronal cells from apoptosis by Hsp27 delivered with a herpes simplex virus-based vector. J. Biol. Chem. 274, 5061–5069.
Wanker E. E. (2000) Protein aggregation in Huntington’s disease and Parkinson’s disease: implications for pathology. Mol. Med. Today 6, 387–391.
Warrick J. M., Chan H. Y., Gray-Board G. L., Chai Y., Paulson H. L., and Bonini N. M. (1999) Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat. Genet. 23, 425–428.
Wellington C. L. and Hayden M. R. (1997) Of molecular interactions, mice and mechanisms: new insights into Huntington’s disease. Curr. Opin. Neurol. 10, 291–298.
Wellington C. L., Ellerby L. M., Hackam A. S., Margolis R. L., Trifiro M. A., Singaraja R., et al. (1998) Caspase cleavage of gene products associated with triplet expansion disorders generates truncated fragments containing the polyglutamine tract. J. Biol. Chem. 273, 9158–9167.
Wellington C. L., Singaraja R., Ellerby L., Savill J., Roy S., Leavitt B., et al. (2000) Inhibiting caspase cleavage of huntingtin reduces toxicity and aggregate formation in neuronal and nonneuronal cells. J. Biol. Chem. 275, 19831–19838.
Wen F. C., Li Y. H., Tsai H. F., Lin C. H., Li C., Liu C. S., et al. (2003 Down-regulation of heat shock protein 27 in neuronal cells and non-neuronal cells expressing mutant ataxin-3. FEBS Lett. 546, 307–314.
Wickner S., Maurizi M. R., and Gottesman S. (1999) Post-translational quality control: folding, refolding, and degrading proteins. Science 286, 1888–1893.
Wiederkehr T., Bukau B., and Buchberger A. (2002) Protein turnover: a CHIP programmed for proteolysis. Curr. Biol. 12, R26-R28.
Wu Z. L., O’Kane T. M., Scott R. W., Savage M. J., and Bozyczko-Coyne D. (2002) Protein tyrosine phosphatases are up-regulated and participate in cell death induced by polyglutamine expansion. J. Biol. Chem. 277, 44208–44213.
Wyttenbach A., Carmichael J., Swartz J., Furlong R. A., Narain Y., Rankin J., and Rubinsztein D. C. (2000) Effects of heat shock, heat shock protein 40 (HDJ-2) and proteasome inhibition on protein aggregation in cellular models of Huntington’s disease. Proc. Natl. Acad. Sci. USA 97, 2898–2903.
Wyttenbach A., Sauvageot O., Carmichael J., Diaz-Latoud C., Arrigo A. P., and Rubinsztein D. C. (2002) Heat shock protein 27 prevents cellular polyglutamine toxicity and suppresses the increase of reactive oxygen species caused by huntingtin. Hum. Mol. Genet. 11, 1137–1151.
Wyttenbach A., Swartz J., Kita H., Thykjaer T., Carmichael J., Bradley J., et al. (2001) Polyglutamine expansions cause decreased CRE-mediated transcription and early gene expression changes prior to cell death in a inducible cell model of Huntington’s disease. Hum. Mol. Genet. 10, 1829–1845.
Xanthoudakis S., Roy S., Rasper D., Hennessey T., Aubin Y., Cassady R., et al. (1999) Hsp60 accelerates the maturation of pro-caspase 3 by upstream activator proteases during apoptosis. EMBO J. 18, 2049–2056.
Xue L., Fletcher G. C., and Tolkovsky A. M. (1999) Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Mol. Cell. Neurosci. 14, 180–198.
Yamamoto A., Lucas J. J., and Hen R. (2000) Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell 101, 57–66.
Yan L. J., Christians E. S., Liu L., Xiao X., Sohal R. S., and Benjamin I. J. (2002) Mouse heat shock transcription factor 1 deficiency alters cardiac redox homeostasis and increases mitochondrial oxidative damage. EMBO J. 21, 5164–5172.
Yang W., Dunlap J. R., Andrews R. B., and Wetzel R. (2002) Aggregated polyglutamine peptides delivered to nuclei are toxic to mammalian cells. Hum. Mol. Genet. 11, 2905–2917.
Yasuda S., Inoue K., Hirabayashi M., Higashiyama H., Yamamoto Y., and Fuyuhiro H. (1999) Triggering of neuronal cell death by accumulation of activated SEK1 on nuclear polyglutamine aggregations in PML bodies. Genes Cells 4, 743–756.
Zabel C., Chamrad D. C., Priller J., Woodman B., Meyer H. E., Bates G. P., and Klose J. (2002) Alterations in the mouse and human proteome caused by Huntington’s disease. Mol. Cell. Proteomics 15, 366–375.
Zander C., Takahashi J., El Hachimi K. H., Fujigasaki H., Albanese V., Lebre A. S., et al. (2001) Similarities between spinocerebellar ataxia type 7 (SCA7) cell models and human brain: proteins recruited in inclusions and activation of caspase-3. Hum. Mol. Genet. 10, 2569–2579.
Zemskov E. A., Jana N. R., Kurosawa M., Miyazaki H., Sakamoto N., Nekooki M., and Nukina N. (2003) Pro-apoptotic protein kinase C delta is associated with intranuclear inclusions in a transgenic model of Huntington’s disease. J. Neurochem. 87, 395–406.
Zeron M. M., Hansson O., Chen N., Wellington C. L., Leavitt B. R., Brundin P., et al. (2002) Increased sensitivity to N-methyl-D-aspartate receptor-mediated excitotoxicity in a mouse model of Huntington’s disease. Neuron 33, 849–860.
Zhou H., Cao F., Wang Z., Yu Z. X., Nguyen H. P., Evans J., Li S. H., and Li X. (2003) Huntingtin forms toxic NH2-terminal fragment complexes that are promoted by the age-dependent decrease in proteasome activity. J. Cell. Biol. 163, 109–118.
Zhou H., Li S. H., and Li X. J. (2001) Chaperone suppression of cellular toxicity of huntingtin is independent of polyglutamine aggregation. J. Biol. Chem. 276, 48417–48424.
Zoghbi H. Y. and Orr H. T. (2000) Glutamine repeats and neurodegeneration. Annu. Rev. Neurosci. 23, 217–247.
Zuccato C., Ciammola A., Rigamonti D., Leavitt B. R, Goffredo D., Conti L., et al. (2001) Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science 293, 493–498.
Zuccato C., Tartari M., Crotti A., Goffredo D., Valenza M., Conti L., et al. (2003) Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat. Genet. 35, 76–83.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wyttenbach, A. Role of heat shock proteins during polyglutamine neurodegeneration. J Mol Neurosci 23, 69–95 (2004). https://doi.org/10.1385/JMN:23:1-2:069
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1385/JMN:23:1-2:069