Abstract
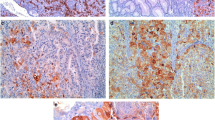
HLA-G belongs to the nonclassical MHC class Ib group of molecules and has been implicated in mediating immune-responsiveness in various cancerous and non-cancerous cell types. We have examined HLA-G expression in a number of human gastrointestinal malignancies, including pancreatic ductal adenocarcinoma, ampullary cancer, biliary cancer, and colorectal cancer by immunolabeling analysis. We used indices of <5% (negative), 6–25%, 26–50%, 51–75%, and >75% (diffuse) to subclassify lesions based on percentage of positive cell labeling. Across all cancer subtypes, 52–79% of lesions demonstrated expression of HLA-G, with up to 33% of lesions demonstrating diffuse (>75%) expression. In addition, we utilized the neoplastic progression model of colorectal cancer to evaluate HLA-G protein expression in normal colon, tubulovillous adenomas, invasive cancer, and liver metastases arising from colorectal cancer. Focal HLA-G expression was detected in regions of normal colon adjacent to sites of adenomatous and cancerous lesions, as well as in all stages of cancer progression. Overall, the percentage of diffusely (>75%) labeled lesions appeared increased in preneoplastic and neoplastic conditions, as compared to normal colon. Specifically, tubulovillous adnenomas demonstrated pronounced diffuse labeling in 58% of lesions examined. No correlation with HLA-G expression and CD4+ or CD8+ T cells was identified. We propose that HLA-G expression is upregulated in a large percentage of gastrointestinal lesions and may serve to mediate immuneresponsiveness in certain instances.
Similar content being viewed by others
References
O’Callaghan CA, Bell JI, Structure and function of the human MHC class Ib molecules HLA-E, HLA-F and HLA-G. Immunol Rev 1998;163:129–138.
Marin R, Ruiz-Cabello F, Pedrinaci S, et al. Analysis of HLA-E expression in human tumors. Immunogenetics 2003;54:767–775.
Carosella ED, Moreau P, Aractingi S, Rouas-Freiss N. HLA-G: a shield against inflammatory aggression. Trends Immunol 2001;22:553–555.
Amiot L, Onno M, Drenou B, et al. Distribution of HLA-G alternative mRNAs including soluble forms in normal lymphocytes and in lymphoid cell-derived leukemia. Eur J Immunogenet 1996;23:311–320.
Onno M, Guillaudeux T, Amiot L, et al. The HLA-G gene is expressed at a low mRNA level in different human cells and tissues. Hum Immunol 1994;41:79–86.
Singer G, Kurman RJ, McMaster MT, Shih Ie M. HLA-G immunoreactivity is specific for intermediate trophoblast in gestational trophoblastic disease and can serve as a useful marker in differential diagnosis. Am J Surg Pathol 2002;26:914–920.
Urosevic M, Kurrer MO, Kamarashev J, et al. Human leukocyte antigen G up-regulation in lung cancer associates with high-grade histology, human leukocyte antigen class I loss and interleukin-10 production. Am J Pathol 2001;159:817–824.
Pangault C, Amiot L, Caulet-Maugendre S, et al. HLA-G protein expression is not induced during malignant transformation. Tissue Antigens 1999;53:335–346.
Seliger B, Abken H, Ferrone S. HLA-G and MIC expression in tumors and their role in anti-tumor immunity. Trends Immunol 2003;24:82–87.
Urosevic M, Willers J, Mueller B, et al. HLA-G protein up-regulation in primary cutaneous lymphomas is associated with interleukin-10 expression in large cell T-cell lymphomas and indolent B-cell lymphomas. Blood 2002;99:609–617.
Wiendl H, Mitsdoerffer M, Hofmeister V, et al. A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J Immunol 2002;168:4772–4780.
Paul P, Cabestre FA, Ibrahim EC, et al. Identification of HLA-G7 as a new splice variant of the HLA-G mRNA and expression of soluble HLA-G5, -G6, and -G7 transcripts in human transfected cells. Hum Immunol 2000;61:1138–1149.
Fournel S, Aguerre-Girr M, Huc X, et al. Cutting edge: soluble HLA-G1 triggers CD95/CD95 ligand-mediated apoptosis in activated CD8+ cells by interacting with CD8. J Immunol 2000;164:6100–6104.
Riteau B, Rouas-Freiss N, Menier C, et al. HLA-G2,-G3, and -G4 isoforms expressed as nonmature cell surface glycoproteins inhibit NK and antigen-specific CTL cytolysis. J Immunol 2001;166:5018–5026.
Riteau B, Menier C, Khalil-Daher I, et al. HLA-G inhibits the allogeneic proliferative response. J Reprod Immunol 1999;43:203–211.
Bainbridge DR, Ellis SA, Sargent IL. HLA-G suppresses proliferation of CD4(+) T-lymphocytes. J Reprod Immunol 2000;48:17–26.
van Heek NT, Meeker AK, Kern SE, et al. Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol 2002;161:1541–1547.
Carosella ED, Paul P, Moreau P, Rouas-Freiss N. HLA-G and HLA-E: fundamental and pathophysiological aspects. Immunol Today 2000;21:532–534.
Contini P, Ghio M, Poggi A, et al. Soluble HL A-A,-B,-C and -G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur J Immunol 2003;33:125–134.
Marchal-Bras-Goncalves R, Rouas-Freiss N, Connan F, et al. Asoluble HLA-G protein that inhibits natural killer cell-mediated cytotoxicity. Transplant Proc 2001;33:2355–2359.
Paul P, Rouas-Freiss N, Khalil-Daher I, et al. HLA-G expression in melanoma: a way for tumor cells to escape from immunosurveillance. Proc Natl Acad Sci USA 1998;95:4510–4515.
Singer G, Rebmann V, Chen Y-C, et al. HLA-G is potential tumor marker in malignant ascites. Clin Cancer Res 2003;9:4460–4464.
Fukushima Y, Oshika Y, Nakamura M, et al. Increased expression of human histocompatibility leukocyte antigen-G in colorectal cancer cells. Int J Mol Med 1998;2:349–351.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hansel, D.E., Rahman, A., Wilentz, R.E. et al. HLA-G upregulation in pre-malignant and malignant lesions of the gastrointestinal tract. Int J Gastrointest Canc 35, 15–23 (2005). https://doi.org/10.1385/IJGC:35:1:015
Issue Date:
DOI: https://doi.org/10.1385/IJGC:35:1:015