Abstract
Purose. Determine the safety and efficacy of twice weekly gemcitabine and concurrent radiation to the upper abdomen followed by weekly gemcitabine in patients with surgically staged, locally advanced pancreatic cancer.
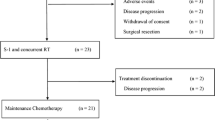
Methods. Patients with surgically staged, locally advanced, nonmetastatic adenocarcinoma of the pancreas were treated with intravenous gemcitabine administered twice weekly (40 mg/m2/d) for 5 wk concurrent with upper abdominal radiation (50.4 Gy in 180 cGy daily fractions over 5.5 wk). At the completion of the chemoradiation, patients without disease progression were given gemcitabine (1000 mg/m2) weekly for five cycles. Each cycle consisted of 3 wk of treatment followed by 1 wk without treatment. Disease progression and response were assessed at 6- to 8-wk intervals.
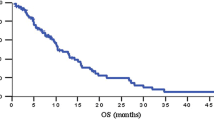
Results. From February through December 1999, 43 patients were entered into this phase II trial, 39 of whom were evaluable for treatment response. The median age was 59 yr (range: 39–84 yr); there were 18 males (47%) in the study. Grade III and IV hematologic toxicity occurred in 48 and 21% of patients, respectively, and was primarily leukocytopenia and neutropenia. Grade III and IV gastrointestinal toxicities occurred in 31 and 10% of patients, respectively. There was one death attributed to sepsis. The concurrent gemcitabine and radiation portion of the study was completed without treatment interruptions in 56% of patients. The overall median survival was 8.2 mo and the median survival in the 44% of patients demonstrating a sustained CA-19-9 response was 13.5 mo. Only six patients experienced local regional progression as their first site of failure. Two patients (5%) were still alive at 35 and 41 mo posttreatment.
Conclusions. These results confirm the feasibility of twice weekly gemcitabine and radiation for the treatment of pancreatic cancer. Although this treatment strategy produced good local regional control, this did not result in a survival advantage. Stratifying patients by performance status and CA-19-9 response in future trials may be of value.
Similar content being viewed by others
References
Douglass HO, Jr. Adjuvant therapy for pancreatic cancer. World J Surg 1995;19:270–274.
Connolly MM, Dawson PJ, Michelassi F. Survival in 1001 patients with carcinoma of the pancreas. Ann Surg 1987;206:366–373.
Gastrointestinal Tumor Study Group. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation+5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer 1981;48:1705–1710.
Gastrointestinal Tumor Study Group. Radiation therapy combined with adriamycin or 5-fluorouracil for the treatment of locally unresectable pancreatic carcinoma. Cancer 1985;56:2563–2568.
Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst 1988;80:751–755.
Klaassen DJ, MacIntyre JM, Catton GE. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil—an Eastern Cooperative Oncology Group study. J Clin Oncol 1985;3:373–378.
Carmichael J, Fink U, Russell RC. Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer 1996;73:101–105.
Casper ES, Green MR, Kelsen DP. Phase II trial of gemcitabine (2, 2′-difluorodeoxycytidine) in patients with adeno-carcinoma of the pancreas. Invest New Drugs 1994;12:29–34.
Burris HA, 3rd, Moore MJ, Andersen J. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial [see comments]. J Clin Oncol 1997;15:2403–2413.
Lawrence TS, Chang EY, Hahn TM. Radiosensitization of pancreatic cancer cells by 2′,2′-difluoro-2′-deoxycytidine. Int J Radiat Oncol Biol Phys 1996;34:867–872.
Lawrence TS, Chang EY, Hahn TM. Delayed radiosensitization of human colon carcinoma cells after a brief exposure to 2′,2′-difluoro-2′-deoxycytidine (gemcitabine). Clin Cancer Res 1997;3:777–782.
Lawrence TS, Eisbruch A, Shewach DS. Gemcitabine-mediated radiosensitization. Semin Oncol 1997;24:S724-S728.
Lawrence TS, Eisbruch A, McGinn CJ. Radiosensitization by gemcitabine. Oncology (Huntingt) 1999;13:55–60.
Shewach DS, Lawrence TS. Radiosensitization of human tumor cells by gemcitabine in vitro. Semin Oncol 1995;22:68–71.
Shewach DS, Lawrence TS. Radiosensitization of human solid tumor cell lines with gemcitabine. Semin Oncol 1996;23:65–71.
Blackstock AW, Lightfoot H, Kwock L. Gemcitabine: in vitro and in vivo evidence of its radiation sensitizing activity and studies using fluorine-19 magnetic resonance spectroscopy to determine the optimal dosing schedule—pre-clinical observations relevent to gemcitabine clinical trials. Int J Radiat Oncol Bio Phys 1997;39:205.
Gandhi V, Plunkett W. Modulatory activity of 2′,2′-difluorodeoxycytidine on the phosphorylation and cytotoxicity of arabinosyl nucleosides. Cancer Res 1990;50:3675–3680.
Boven E, Schipper H, Erkelens CA. The influence of the schedule and the dose of gemcitabine on the anti-tumour efficacy in experimental human cancer. Br J Cancer 1993;68:52–56.
Shewach DS, Hahn TM, Chang E. Metabolism of 2′,2′-difluoro-2′-deoxycytidine and radiation sensitization of human colon carcinoma cells. Cancer Res 1994;54:3218–3223.
Grunewald R, Abbruzzese JL, Tarassoff P. Saturation of 2′,2′-difluorodeoxycytidine 5′-triphosphate accumulation by mononuclear cells during a phase I trial of gemcitabine. Cancer Chemother Pharmacol 1991;27:258–262.
Blackstock AW, Lightfoot H, Case LD. Tumor uptake and elimination of 2′,2′-difluoro-2′-deoxycytidine (gemcitabine) after deoxycytidine kinase gene transfer: correlation with in vivo tumor response. Clin Cancer Res 2001;7:3263–3268.
Fields MT, Eisbruch A, Normolle D. Radiosensitization produced in vivo by once- vs. twice-weekly 2′2′-difluoro-2′-deoxycytidine (gemcitabine). Int J Radiat Oncol Biol Phys 2000;47:785–791.
Mason KA, Milas L, Hunter NR. Maximizing therapeutic gain with gemcitabine and fractionated radiation. Int J Radiat Oncol Biol Phys 1999;44:1125–1135.
Eisbruch A, Shewach DS, Bradford CR. Radiation concurrent with gemcitabine for locally advanced head and neck cancer: a phase I trial and intracellular drug incorporation study. J Clin Oncol 2001;19:792–799.
Blackstock AW, Bernard SA, Richards F. Phase I trial of twice-weekly gemcitabine and concurrent radiation in patients with advanced pancreatic cancer. J Clin Oncol 1999;17:2208–2212.
McGinn CJ, Lawrence TS. Recent advances in the use of radiosensitizing nucleoside. Semin Radiat Oncol 2001;11:270–280.
Rosier JF, Beauduin M, Bruniaux M. The effect of 2′-2′ difluorodeoxycytidine (dFdC, gemcitabine) on radiation-induced cell lethality in two human head and neck squamous carcinoma cell lines differing in intrinsic radiosensitivity. Int J Radiat Biol 1999;75:245–251.
Talamonti MS, Catalano PJ, Vaughn DJ. Eastern Cooperative Oncology Group phase I trial of protracted venous infusion fluorouracil plus weekly gemcitabine with concurrent radiation therapy in patients with locally advanced pancreas cancer: aregimen with unexpected early toxicity. J Clin Oncol 2000;18:3384–3389.
Wolff RA, Evans DB, Gravel DM. Phase I trial of gemcitabine combined with radiation for the treatment of locally advanced pancreatic adenocarcinoma. Clin Cancer Res 2001;7:2246–2253.
Crane CH, Janjan NA, Evans DB. Toxicity and efficacy of concurrent gemcitabine and radiotherapy for locally advanced pancreatic cancer. Int J Pancreatol 2001;29:9–18.
McGinn CJ, Zalupski MM, Shureiqi I. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 2001;19:4202–4208.
Yavuz AA, Aydin F, Yavuz MN. Radiation therapy and concurrent fixed dose amifostine with escalating doses of twiceweekly gemcitabine in advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2001;51:974–981.
Pipas JM, Mitchell SE, Barth RJ. Phase I study of twice-weekly gemcitabine and concomitant external-beam radiotherapy in patients with adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys 2001;50:1317–1322.
Lund B, Ryberg M, Petersen PM. Phase II study of gemcitabine (2′,2′-diflurodeoxycytidine) given as a twice weekly schedule to previously untreated patients with nonsmall cell lung cancer. Ann Oncol 1994;5:852–853.
Martenson JA, Vigliotti AP, Pitot HC. A phase I study of radiation therapy and twice-weekly gemcitabine and cisplatin in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2003;55(5):1305–1310.
Rich T, Harris J, Abrams R. A phase II study of external irradiation and weekly paclitaxel for non-metastatic, unresectable pancreatic cancer: a preliminary report of RTOG Protocol 98-12. Int J Radiat Bio Phys 2002;51:29–30.
Gogas H, Lofts FJ, Evans TR. Are serial measurements of CA19-9 useful in predicting response to chemotherapy in patients with inoperable adenocarcinoma of the pancreas? Br J Cancer 1998;77:325–328.
Halm U, Schumann T, Schiefke I. Decrease of CA 19-9 during chemotherapy with gemcitabine predicts survival time in patients with advanced pancreatic cancer. Br J Cancer 2000;82:1013–1016.
Ishii H, Okada S, Stao T. CA 19-9 in evaluating the response to chemotherapy in advanced pancreatic cancer. Hepatogastroenterology 1997;44:279–283.
Willett CG, Daly WJ, Warshaw AL. CA 19-9 is an index of response to neoadjunctive chemoradiation therapy in pancreatic cancer. Am J Surg 1996;172:350–352.
Komaki R, Hansen R, Cox JD. Phase I-II study of prophylactic hepatic irradiation with local irradiation and systemic chemotherapy for adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys 1998;15:1447–1452.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
William Blackstock, A., Tepper, J.E., Niedwiecki, D. et al. Cancer and leukemia group B (CALGB) 89805. Int J Gastrointest Canc 34, 107–116 (2003). https://doi.org/10.1385/IJGC:34:2-3:107
Issue Date:
DOI: https://doi.org/10.1385/IJGC:34:2-3:107