Abstract
Background
Mesenteric lymph node (MLN) involvement is often observed in ovarian cancer (OC) with rectosigmoid invasion. This study aimed to investigate the clinical significance of MLN involvement in the pattern of liver metastasis in patients with OC.
Methods
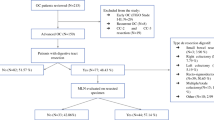
We included 85 stage II–IV OC patients who underwent primary or interval debulking surgery. Twenty-seven patients underwent rectosigmoid resection, whose status of MLN involvement was judged from hematoxylin and eosin (H&E) staining of resected specimens. The prognostic significance of clinicopathological characteristics, including MLN involvement, was evaluated using univariate and multivariate analyses.
Results
MLN involvement was detected in 14/85 patients with stage II–IV OC. Residual tumor status, cytology of ascites, and MLN involvement were independent prognostic factors for progression-free survival (PFS; p = 0.033, p = 0.014, and p = 0.008, respectively). When patients were classified into three groups (no MLN, one MLN, two or more MLNs), the number of MLNs involved corresponded to three distinct groups in PFS (p = 0.001). The 3-year cumulative incidence of liver metastasis of patients with MLN involvement was significantly higher than that of patients without MLN involvement (61.1% vs. 8.9%, p < 0.001). MLN involvement was significantly associated with liver metastasis of hematogenous origin (p < 0.001) compared with peritoneal disseminated origin.
Conclusion
MLN involvement is an important prognostic factor in OC, predicting poor prognosis and liver metastasis of hematogenous origin.




Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Bristow RE, Montz FJ, Lagasse LD, Leuchter RS, Karlan BY. Survival impact of surgical cytoreduction in stage IV epithelial ovarian cancer. Gynecol Oncol. 1999;72:278–87.
Eisenkop SM, Spirtos NM. Procedures required to accomplish complete cytoreduction of ovarian cancer: Is there a correlation with “biological aggressiveness” and survival? Gynecol Oncol. 2001;82:435–41.
Chang SJ, Hodeib M, Chang J, Bristow RE. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: A meta-analysis. Gynecol Oncol. 2013;130:493–8.
Scarabelli C, Gallo A, Franceschi S, et al. Primary cytoreductive surgery with rectosigmoid colon resection for patients with advanced epithelial ovarian carcinoma. Cancer. 2000;88:389–97.
Jaeger W, Ackermann S, Kessler H, Katalinic A, Lang N. The effect of bowel resection on survival in advanced epithelial ovarian cancer. Gynecol Oncol. 2001;83:286–91.
Obermair A, Hagenauer S, Tamandl D, et al. Safety and efficacy of low anterior en bloc resection as part of cytoreductive surgery for patients with ovarian cancer. Gynecol Oncol. 2001;83:115–20.
Mourton SM, Temple LK, Abu-Rustum NR, et al. Morbidity of rectosigmoid resection and primary anastomosis in patients undergoing primary cytoreductive surgery for advanced epithelial ovarian cancer. Gynecol Oncol. 2005;99:608–14.
Aletti GD, Podratz KC, Jones MB, Cliby WA. Role of rectosigmoidectomy and stripping of pelvic peritoneum in outcomes of patients with advanced ovarian cancer. J Am Coll Surg. 2006;203:521–6.
Estes JM, Leath CA 3rd, Straughn JM Jr, et al. Bowel resection at the time of primary debulking for epithelial ovarian carcinoma: outcomes in patients treated with platinum and taxane-based chemotherapy. J Am Coll Surg. 2006;203:527–32.
Park JY, Seo SS, Kang S, et al. The benefits of low anterior en bloc resection as part of cytoreductive surgery for advanced primary and recurrent epithelial ovarian cancer patients outweigh morbidity concerns. Gynecol Oncol. 2006;103:977–84.
Richardson DL, Mariani A, Cliby WA. Risk factors for anastomotic leak after recto-sigmoid resection for ovarian cancer. Gynecol Oncol. 2006;103:667–72.
Salani R, Zahurak ML, Santillan A, Giuntoli RL 2nd, Bristow RE. Survival impact of multiple bowel resections in patients undergoing primary cytoreductive surgery for advanced ovarian cancer: a case-control study. Gynecol Oncol. 2007;107:495–9.
Peiretti M, Bristow RE, Zapardiel I, et al. Rectosigmoid resection at the time of primary cytoreduction for advanced ovarian cancer. A multi-center analysis of surgical and oncological outcomes. Gynecol Oncol. 2012;126:220–3.
Giorda G, Gadducci A, Lucia E, et al. Prognostic role of bowel involvement in optimally cytoreduced advanced ovarian cancer: a retrospective study. J Ovarian Res. 2014;7:72.
Derlatka P, Sienko J, Grabowska-Derlatka L, et al. Results of optimal debulking surgery with bowel resection in patients with advanced ovarian cancer. World J Surg Oncol. 2016;14:58.
Philip CA, Pelissier A, Bonneau C, Hequet D, Rouzier R, Pouget N. Impact of neoadjuvant chemotherapy on the rate of bowel resection in advanced epithelial ovarian cancer. Anticancer Res. 2016;36:4865–71.
Plotti F, Montera R, Aloisi A, et al. Total rectosigmoidectomy versus partial rectal resection in primary debulking surgery for advanced ovarian cancer. Eur J Surg Oncol. 2016;42:383–90.
Kim M, Suh DH, Park JY, et al. Survival impact of low anterior resection in patients with epithelial ovarian cancer grossly confined to the pelvic cavity: a Korean multicenter study. J Gynecol Oncol. 2018;29:e60.
Tozzi R, Casarin J, Baysal A, et al. Bowel resection rate but not bowel related morbidity is decreased after interval debulking surgery compared to primary surgery in patents with stage IIIC-IV ovarian cancer. J Gynecol Oncol. 2019;30:e25.
O’Hanlan KA, Kargas S, Schreiber M, et al. Ovarian carcinoma metastases to gastrointestinal tract appear to spread like colon carcinoma: implications for surgical resection. Gynecol Oncol. 1995;59:200–6.
Salani R, Diaz-Montes T, Giuntoli RL, Bristow RE. Surgical management of mesenteric lymph node metastasis in patients undergoing rectosigmoid colectomy for locally advanced ovarian carcinoma. Ann Surg Oncol. 2007;14:3552–7.
Baiocchi G, Cestari LA, Macedo MP, et al. Surgical implications of mesenteric lymph node metastasis from advanced ovarian cancer after bowel resection. J Surg Oncol. 2011;104:250–4.
Gouy S, Goetgheluck J, Uzan C, Duclos J, Duvillard P, Morice P. Prognostic factors for and prognostic value of mesenteric lymph node involvement in advanced-stage ovarian cancer. Eur J Surg Oncol. 2012;38:170–5.
Di Giorgio A, Cardi M, Biacchi D, et al. Depth of colorectal-wall invasion and lymph-node involvement as major outcome factors influencing surgical strategy in patients with advanced and recurrent ovarian cancer with diffuse peritoneal metastases. World J Surg Oncol. 2013;11:64.
Kumagai K, Okamura T, Toyoda M, Senzaki H, Watanabe C, Ohmichi M. Rectal lymph node metastasis in recurrent ovarian carcinoma: essential role of 18F-FDG PET/CT in treatment planning. World J Surg Oncol. 2013;11:184.
Gallotta V, Fanfani F, Fagotti A, et al. Mesenteric lymph node involvement in advanced ovarian cancer patients undergoing rectosigmoid resection: prognostic role and clinical considerations. Ann Surg Oncol. 2014;21:2369–75.
Berretta R, Capozzi VA, Sozzi G, et al. Prognostic role of mesenteric lymph nodes involvement in patients undergoing posterior pelvic exenteration during radical or supra-radical surgery for advanced ovarian cancer. Arch Gynecol Obstet. 2018;297:997–1004.
Amin MB, Edge S, Greene F, et al editors. AJCC cancer staging manual. 8th edn. New York: Springer International; 2017.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology-392rectal cancer (version 6. 2020). Available at: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed 6 Nov 2020.
Lim MC, Kang S, Lee KS, et al. The clinical significance of hepatic parenchymal metastasis in patients with primary epithelial ovarian cancer. Gynecol Oncol. 2009;112:28–34.
O’Neill AC, Somarouthu B, Tirumani SH, et al. Patterns and prognostic importance of hepatic involvement in patients with serous ovarian cancer: a single-institution experience with 244 patients. Radiology. 2017;282:160–70.
Chi DS, Temkin SM, Abu-Rustum NR, Sabbatini P, Jarnagin WR, Blumgart LH. Major hepatectomy at interval debulking for stage IV ovarian carcinoma: a case report. Gynecol Oncol. 2002;87:138–42.
Lee JH, Kim KS, Chung CW, Park YN, Kim BR. Hepatic resection of metastatic tumor from serous cystadenocarcinoma of the ovary. J Korean Med Sci. 2002;17:415–8.
Merideth MA, Cliby WA, Keeney GL, Lesnick TG, Nagorney DM, Podratz KC. Hepatic resection for metachronous metastases from ovarian carcinoma. Gynecol Oncol. 2003;89:16–21.
Bacalbasa N, Dima S, Brasoveanu V, et al. Liver resection for ovarian cancer liver metastases as part of cytoreductive surgery is safe and may bring survival benefit. World J Surg Oncol. 2015;13:235.
Wang M, Zhou J, Zhang L, et al. Surgical treatment of ovarian cancer liver metastasis. Hepatobiliary Surg Nutr. 2019;8:129–37.
Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–96.
Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83.
Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16:928–36.
Tewari KS, Burger RA, Enserro D, et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer. J Clin Oncol. 2019;37:2317–28.
Rinderknecht M, Detmar M. Molecular mechanisms of lymph node metastasis. In: Stacker SA, Achen MG, editors. Lymphangiogenesis in cancer metastasis: cancer metastasis—biology and treatment 13. Dordrecht: Springer; 2009. p. 55–82.
Kumar V, Abbas AK, Aster JC. Robbins basic pathology. 10th edn. Pennsylvania: Elsevier; 2019.
Funding
This research was supported by JSPS KAKENHI Grant Number JP19K09116 (MN, YS, HK, TW, KN, TE, KY, and KT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Kana Tanaka, Yoshifumi Shimada, Koji Nishino, Kosuke Yoshihara, Masato Nakano, Hitoshi Kameyama, Takayuki Enomoto and Toshifumi Wakai has nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tanaka, K., Shimada, Y., Nishino, K. et al. Clinical Significance of Mesenteric Lymph Node Involvement in the Pattern of Liver Metastasis in Patients with Ovarian Cancer. Ann Surg Oncol 28, 7606–7613 (2021). https://doi.org/10.1245/s10434-021-09899-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-09899-8