Abstract
Introduction
Incidence of peritoneal carcinomatosis (PC) after curative resection of stage II and III colon cancer varies widely. Although certain features are considered high risk for PC, the impact of these features on PC incidence is unclear.
Methods
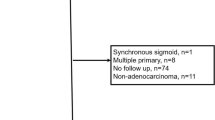
A retrospective analysis was performed on patients ≥ 18 years old with resected stage II and III colonic adenocarcinoma treated at two academic institutions from 2007 to 2018. Clinicopathologic features, treatment and outcomes data were recorded. Patients with reported high-risk features (pT3N0-2 with mucinous/signet ring components, pT4, pN1c, perforation) were identified. The remaining stage II and III patients were used for comparison.
Results
Of 219 eligible patients, 93/219 (42.5%) were stage II and 126/219 (57.5%) were stage III. Median follow-up time was 25 (1–146) months. Adjuvant systemic treatment was administered to 133/219 (60.7%) patients. Overall incidence of PC was 14/219 (6.4%) and the median time to PC was 18 (1–37) months. The high-risk and comparison groups contained 113 and 106 patients, respectively. Incidence of PC was significantly different between groups (high-risk 9.7% vs comparison 2.8%, p = 0.04). Median time to PC was not significantly different between the groups [high-risk 17 (1–37) months vs comparison 20 (7–36) months, p = 0.88].
Conclusion
Overall PC incidence in patients with resected stage II and III colon cancer was 6.4%. Although the high-risk group developed PC at a significantly higher rate, the rate of PC in this group was still below 10%. The results of this study represent real-world rates of PC and should be taken into account when designing future studies.
Similar content being viewed by others
References
Franko J, Shi Q, Meyers JP, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17(12):1709–19.
Baratti D, Kusamura S, Pietrantonio F, Guaglio M, Niger M, Deraco M. Progress in treatments for colorectal cancer peritoneal metastases during the years 2010–2015. A systematic review. Crit Rev Oncol Hematol. 2016;100:209–22.
Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22(16):3284–92.
Quenet F, Elias D, Roca L, et al. A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. J Clin Oncol. 2018;36(18):LBA3503.
Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15(9):2426–32.
Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737–43.
Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89(12):1545–50.
Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP. Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg. Feb 2006;243(2):212-222.
Baguena G, Pellino G, Frasson M, et al. Prognostic impact of pT stage and peritoneal invasion in locally advanced colon cancer. Dis Colon Rectum. 2019;62(6):684–93.
Enblad M, Graf W, Birgisson H. Risk factors for appendiceal and colorectal peritoneal metastases. Eur J Surg Oncol. 2018;44(7):997–1005.
Hugen N, van de Velde CJ, de Wilt JH, Nagtegaal ID. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol. 2014;25(3):651–7.
Leung V, Huang N, Liauw W, Morris DL. High risk features of primary colorectal carcinomas which subsequently undergo peritonectomy. Eur J Surg Oncol. Jun 2016;42(6):836-840.
Tajiri K, Sudou T, Fujita F, Hisaka T, Kinugasa T, Akagi Y. Clinicopathological and corresponding genetic features of colorectal signet ring cell carcinoma. Anticancer Res. 2017;37(7):3817–23.
Mayanagi S, Kashiwabara K, Honda M, et al. Risk factors for peritoneal recurrence in stage II to III colon cancer. Dis Colon Rectum. 2018;61(7):803–8.
Nagata H, Ishihara S, Hata K, et al. Survival and prognostic factors for metachronous peritoneal metastasis in patients with colon cancer. Ann Surg Oncol. May 2017;24(5):1269-1280.
Klaver CEL, Wisselink DD, Punt CJA, et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): a multicentre, open-label, randomised trial. Lancet Gastroenterol Hepatol. 2019;4(10):761–70.
Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. Jun 3 2004;350(23):2343-2351.
Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–16.
Goere D, Glehen O, Quenet F, et al. Results of a randomized phase 3 study evaluating the potential benefit of a second-look surgery plus HIPEC in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP- NTC01226394). J Clin Oncol. 2018;36(15_suppl):3531.
Bastiaenen VP, Klaver CEL, Kok NFM, et al. Second and third look laparoscopy in pT4 colon cancer patients for early detection of peritoneal metastases; the COLOPEC 2 randomized multicentre trial. BMC Cancer. 2019;19(1):254.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Pigazzi serves as consultant for Medtronic, Ethicon and Vioptix.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choi, A.H., Farzaneh, C., Kejriwal, N. et al. Rate of Peritoneal Carcinomatosis in Resected Stage II and III Colon Cancer. Ann Surg Oncol 27, 4943–4948 (2020). https://doi.org/10.1245/s10434-020-08689-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08689-y