Abstract
Background
Significant antitumor T-cell responses are generated in vitro when human lymphocytes are stimulated with autologous tumor cells in the presence of bystander cells transfected with CD40L and GM-CSF. Our goal was to test this bystander-based vaccine strategy in vivo in cancer patients with stage IV disease.
Methods
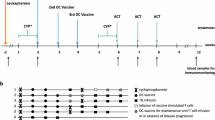
Patients received three intradermal vaccine injections (irradiated autologous tumor cells plus GM.CD40L bystander cells) at 28-day intervals. Patients with no disease progression received three additional vaccines at 4, 12, and 24 months. Patients were monitored for toxicity, tumor response, and tumor-specific immune responses.
Results
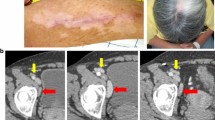
Twenty-one patients received at least three vaccine injections, with no toxicity attributable to the vaccine. Immunohistochemistry of vaccine injection site biopsies with CD1a and CD86 antibodies confirmed recruitment and activation of dendritic cells. There was no tumor regression after vaccination, but many patients had stable disease, including six of ten melanoma patients. Four patients developed tumor-specific T-cell responses on ELISPOT testing. One patient, who had stable disease for 24 months, demonstrated an increase in MART-1-specific T-cells by tetramer analysis after re-immunization; biopsy of the tumor that progressed 2 years after the onset of vaccination revealed a massive peritumoral and intratumoral T-cell infiltrate.
Conclusions
Vaccination of cancer patients with autologous tumor cells and GM.CD40L bystander cells (engineered to express GM-CSF and CD40L) is safe, can recruit and activate dendritic cells, and can elicit tumor-specific T-cell responses. Phase-II trials are underway to evaluate the impact of bystander-based vaccines on melanoma and mantle cell lymphoma.







Similar content being viewed by others
Abbreviations
- APC:
-
antigen-presenting cell
- CD40L:
-
CD40 ligand
- DC:
-
dendritic cell
- DTH:
-
delayed-type hypersensitivity
- ELISA:
-
enzyme-linked immunosorbent assay
- GM-CSF:
-
granulocyte macrophage-colony stimulating factor
- IL:
-
interleukin
- MIP-1:
-
monocyte inflammatory protein-1
- MRD:
-
minimal residual disease
- PBMC:
-
peripheral blood mononuclear cell
- RECIST:
-
Response Evaluation Criteria in Solid Tumors
- TAA:
-
tumor-associated antigen
References
Rosenberg SA. Identification of cancer antigens: impact on development of cancer immunotherapies. Cancer J 2000; 6(Suppl 3):S200–S207
Pittet MJ, Valmori D, Dunbar PR et al. High frequencies of naive Melan-A/MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J Exp Med 1999; 190:705–715
Anichini A, Molla A, Mortarini R et al. An expanded peripheral T cell population to a cytotoxic T lymphocyte (CTL)-defined, melanocyte-specific antigen in metastatic melanoma patients impacts on generation of peptide-specific CTLs but does not overcome tumor escape from immune surveillance in metastatic lesions. J Exp Med 1999; 190:651–667
Jager E, Nagata Y, Gnjatic S et al. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. PNAS (USA) 2000; 97:4760–4765
Dessureault S, Alsarraj M, McCarthy S et al. A GM-CSF/CD40L Producing Cell Augments Anti-tumor T Cell Responses. J Surg Res 2005; 125:173–181
Sotomayor EM, Borrello I, Tubb E et al. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat Med 1999; 5:780–787
Brossart P, Zobywalski A, Grunebach F et al. Tumor necrosis factor alpha and CD40 ligand antagonize the inhibitory effects of interleukin 10 on T-cell stimulatory capacity of dendritic cells. Cancer Res 2000; 60:4485–4492
Pirtskhalaishvili G, Shurin GV, Esche C et al. Cytokine-mediated protection of human dendritic cells from prostate cancer-induced apoptosis is regulated by the Bcl-2 family of proteins. Br J Cancer 2000; 83:506–513
Esche C, Gambotto A, Satoh Y et al. CD154 inhibits tumor-induced apoptosis in dendritic cells and tumor growth. Eur J Immunol 1999; 29:2148–2155
Mach N, Dranoff G. Cytokine-secreting tumor cell vaccines. Curr Opin Immunol 2000; 12:571–575
Nelson WG, Simons JW, Mikhak B, et al. Cancer cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer as vaccines for the treatment of genitourinary malignancies. Cancer Chemotherapy & Pharmacology 2000; 46 Suppl:S67–S72
Simons JW, Jaffee EM, Weber CE, et al. Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte-macrophage colony-stimulating factor gene transfer. Cancer Res 1997; 57:1537–1546
Chang AE, Li Q, Bishop DK, Normolle DP, Redman BD, Nickoloff BJ. Immunogenetic therapy of human melanoma utilizing autologous tumor cells transduced to secrete granulocyte-macrophage colony-stimulating factor. Hum Gene Ther 2000; 11:839–850
Simons JW, Mikhak B, Chang JF et al. Induction of Immunity to Prostate Cancer Antigens: Results of a Clinical Trial of Vaccination with Irradiated Autologous Prostate Tumor Cells Engineered to Secrete Granulocyte-Macrophage Colony-stimulating Factor Using ex Vivo Gene Transfer. Cancer Res 1999; 59:5160–5168
Soiffer R, Lynch T, Mihm M et al. Vaccination with irradiated autologous melanoma cells engineered to secrete human granulocyte-macrophage colony-stimulating factor generates potent antitumor immunity in patients with metastatic melanoma. PNAS (USA) 1998; 95:13141–13146
Kusumoto M, Umeda S, Ikubo A et al. Phase 1 clinical trial of irradiated autologous melanoma cells adenovirally transduced with human GM-CSF gene. Cancer Immunol Immunother 2001; 50:373–381
Chiodoni C, Paglia P, Stoppacciaro A, Rodolfo M, Parenza M, Colombo MP. Dendritic cells infiltrating tumors cotransduced with granulocyte/macrophage colony-stimulating factor (GM-CSF) and CD40 ligand genes take up and present endogenous tumor-associated antigens, and prime naive mice for a cytotoxic T lymphocyte response. J Exp Med 1999; 190:125–133
Borrello I, Sotomayor EM, Cooke S, Levitsky HI. A universal granulocyte-macrophage colony-stimulating factor-producing bystander cell line for use in the formulation of autologous tumor cell-based vaccines. Hum Gene Ther 1999; 10:1983–1991
Dotti G, Savoldo B, Yotnda P, Rill D, Brenner MK. Transgenic expression of CD40 ligand produces an in vivo antitumor immune response against both CD40 (+) and CD40 (-) plasmacytoma cells. Blood 2002; 100:200–207
Andersson LC, Nilsson K, Gahmberg CG. K562—a human erythroleukemic cell line. Int J Cancer 1979; 23:143–147
Dessureault S, Graham FL, Gallinger S. Autologous lymphocyte responses to adenovirus-B7-1-transduced human cancer cells. Cancer Gene Ther 1999; 6:195–208
Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med 1996; 184:747–752
Salazar LG, Disis ML. Cancer vaccines: the role of tumor burden in tipping the scale toward vaccine efficacy. J Clin Oncol 2005; 23:7397–7398
Luiten RM, Kueter EWM, Mooi W et al. Immunogenicity, including vitiligo, and feasibility of vaccination with autologous GM-CSF-transduced tumor cells in metastatic melanoma patients. J Clin Oncol 2005; 23:8978–8991
Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res 2006; 12:878–887
Gribben JG, Ryan DP, Boyajian R et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin Cancer Res 2005; 11:4430–4436
Wheeler CJ, Das A, Liu G, Yu JS, Black KL. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res 2004; 10:5316–5326
Acknowledgments
First and foremost, we wish to thank the patients who participated in this study and the physicians who made it possible for us to share in their care. We thank and acknowledge the services of the Flow Cytometry Core Laboratory, the Cell Therapy Core Facility, and the Clinical Research Unit at the H. Lee Moffitt Cancer Center and Research Institute. We also thank and acknowledge Sandy Livingston of the Histology Laboratory in the Pathology Core Facility at the College of Medicine, University of South Florida, for immunohistochemical staining of the biopsy specimens. We wish to thank the study coordinators (Mary Dunn and Diane Garry) and regulatory specialist (Mary Willis) for their contributions throughout the course of the study. This work was sponsored by the H. Lee Moffitt Cancer Center & Research Institute and supported in part by the Flight Attendant Medical Research Institute. Sophie Dessureault was supported in part by a Society of Surgical Oncology (SSO) James Ewing Young Investigator Award for Clinical Research and in part by the American Society of Clinical Oncology (ASCO) Career Development Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dessureault, S., Noyes, D., Lee, D. et al. A phase-I Trial Using a Universal GM-CSF-producing and CD40L-expressing Bystander Cell Line (GM.CD40L) in the Formulation of Autologous Tumor Cell-based Vaccines for Cancer Patients with Stage IV disease. Ann Surg Oncol 14, 869–884 (2007). https://doi.org/10.1245/s10434-006-9196-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-006-9196-4