Abstract
Purpose
Neoadjuvant therapy (NAT) has been shown to clinically downstage locally advanced breast cancers. This study aimed to determine whether a meaningful change in gene signatures occurs between pre- and post-NAT breast cancers for patients who do not achieve a pathologic complete response.
Methods
The current analysis included women from the prospective Neoadjuvant Breast Registry Symphony Trial who had breast cancer and awaited NAT. MammaPrint and BluePrint (Agendia, Inc., Irvine, CA) assays were performed on pre- and post-NAT breast tumor samples.
Results
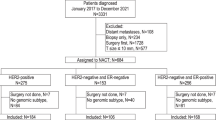
At the completion of NAT, 93 patients with residual disease had their remaining tumor analyzed for MammaPrint and BluePrint. Of 93 patients, 21 switched tumor classification: 16 from high risk (HR) to low risk (LR) and 1 from LR to HR (p < 0.001). Four additional patients switched molecular subtype but remained HR. Although only 17 patients switched in their MammaPrint risk classification, the underlying MPIndex was significantly altered after treatment across all patients (p < 0.001). Additionally, the three BluePrint indices for luminal, human epidermal growth factor receptor 2 (HER2), and basal type also were significantly altered after treatment, in a subtype-dependent manner.
Conclusion
This substudy showed that NAT significantly altered the genomic signature of the patient’s breast cancer compared with the patient’s pretreatment genomic profile. These alterations occurred in a subtype-dependent manner, suggesting that NAT may have either eliminated the most susceptible tumor subclone, leaving the treatment resistant clone with a different genetic signature, or altered molecular characteristics of the original cancer.


Similar content being viewed by others
References
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–72.
Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL–CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242–9.
Kawajiri H, Takashima T, Aomatsu N, et al. Prognostic significance of pathological complete response following neoadjuvant chemotherapy for operable breast cancer. Oncol Lett. 2014;7:663–8.
Perou CM, Borresen-Dale AL. Systems biology and genomics of breast cancer. Cold Spring Harb Perspect Biol. 2011;3:a003293.
Perou CM, Parker JS, Prat A, Ellis MJ, Bernard PS. Clinical implementation of the intrinsic subtypes of breast cancer. Lancet Oncol. 2010;11:718–9; author reply 711–20.
von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–804.
Gluck S, de Snoo F, Peeters J, Stork-Sloots L, Somlo G. Molecular subtyping of early-stage breast cancer identifies a group of patients who do not benefit from neoadjuvant chemotherapy. Breast Cancer Res Treat. 2013;139:759–67.
Krijgsman O, Roepman P, Zwart W, et al. A diagnostic gene profile for molecular subtyping of breast cancer associated with treatment response. Breast Cancer Res Treat. 2012;133:37–47.
Whitworth P, Stork-Sloots L, de Snoo FA, et al. Chemosensitivity predicted by BluePrint 80-gene functional subtype and MammaPrint in the Prospective Neoadjuvant Breast Registry Symphony Trial (NBRST). Ann Surg Oncol. 2014;21:3261–7.
Glas AM, Floore A, Delahaye LJ, et al. Converting a breast cancer microarray signature into a high-throughput diagnostic test. BMC Genomics. 2006;7:278.
Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010;134:e48–72.
Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013.
Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45.
Sapino A, Roepman P, Linn SC, et al. MammaPrint molecular diagnostics on formalin-fixed, paraffin-embedded tissue. J Mol Diagn. 2014;16:190–7.
Gahlaut R, Bennett A, Fatayer H, et al. Effect of neoadjuvant chemotherapy on breast cancer phenotype, ER/PR and HER2 expression: implications for the practising oncologist. Eur J Cancer. 2016;60:40–8.
van de Ven S, Smit VT, Dekker TJ, Nortier JW, Kroep JR. Discordances in ER, PR, and HER2 receptors after neoadjuvant chemotherapy in breast cancer. Cancer Treat Rev. 2011;37:422–30.
Somlo G, Lau SK, Frankel P, et al. Multiple biomarker expression on circulating tumor cells in comparison to tumor tissues from primary and metastatic sites in patients with locally advanced/inflammatory, and stage IV breast cancer, using a novel detection technology. Breast Cancer Res Treat. 2011;128:155–63.
Burstein HJ, Harris LN, Gelman R, et al. Preoperative therapy with trastuzumab and paclitaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for HER2 overexpressing stage II or III breast cancer: a pilot study. J Clin Oncol. 2003;21:46–53.
Guarneri V, Dieci MV, Barbieri E, et al. Loss of HER2 positivity and prognosis after neoadjuvant therapy in HER2-positive breast cancer patients. Ann Oncol. 2013;24:2990–4.
Harris LN, You F, Schnitt SJ, et al. Predictors of resistance to preoperative trastuzumab and vinorelbine for HER2-positive early breast cancer. Clin Cancer Res. 2007;13:1198–207.
Mittendorf EA, Wu Y, Scaltriti M, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res. 2009;15:7381–8.
Barker AD, Sigman CC, Kelloff GJ, Hylton NM, Berry DA, Esserman LJ. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin Pharmacol Ther. 2009;86:97–100.
Acknowledgments
We thank all the women who participated in this study and all the investigators, surgeons, pathologists, and research nurses. The authors also thank Sammy Mee, Divya Malhotra, and Laboratory Operations (Agendia Inc.) for sample processing support.
Conflict of interest
Tina Treece, PhD and Erin Yoder, MS are employed by Agendia, Inc.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Beitsch, P., Whitworth, P., Baron, P. et al. Genomic Impact of Neoadjuvant Therapy on Breast Cancer: Incomplete Response is Associated with Altered Diagnostic Gene Signatures. Ann Surg Oncol 23, 3317–3323 (2016). https://doi.org/10.1245/s10434-016-5329-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-016-5329-6