Abstract
Background
The role of glucose transporter 14 (GLUT-14/SLC2A14) in tumor biology is entirely unknown, and the significance of hypoxia inducible factor 1-alpha (HIF1-α) for gastric adenocarcinoma is controversial. The impact of GLUT-1/SLC2A1 has never been confirmed in a Caucasian cohort.
Methods
Between 1996 and 2007, 124 patients underwent gastrectomy for gastric adenocarcinoma. Tumor sections were incubated with GLUT-1, GLUT-14, and HIF1-α antibodies. Expression was analyzed for correlations with histopathology, marker coexpression, and patient survival by uni- and multivariate analyses.
Results
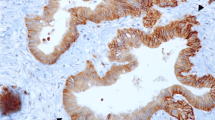
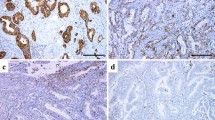
Expressions of GLUT-1, GLUT-14, and HIF1-α were detectable in 50, 77.4, and 27.1 %, respectively. Expression of GLUT-1 was associated with pT-category (p = 0.019), pN-category (p = 0.019), tubular (WHO, p = 0.008), and intestinal (Lauren classification; p = 0.002) histologic subtypes. Expression of GLUT-14 was correlated with pT category (p = 0.043), whereas HIF1-α did not show any correlation with histopathology or survival. The median survival period was 14 months (95 % confidence interval [CI] 9.2–18.8 months) for GLUT-1-positive patients and 55 months (95 % CI 25.8–84.2; p = 0.01) for GLUT-1-negative patients. An inferior prognosis also was seen for GLUT-14-positive cases compared with GLUT-14-negative cases (p = 0.004). Thus, worst survival was seen with both GLUT-1- and GLUT-14-positive expression followed by single-positive and then double-negative cases (p = 0.004). In multivariate analysis including International Union Against Cancer (UICC) stages, R category, Lauren classification, surgery alone versus neoadjuvant/perioperative chemotherapy, and marker expression as covariates, GLUT-1 (p = 0.011) and GLUT-14 (p = 0.025) kept their prognostic independence.
Conclusions
The study findings suggest that detection of GLUT-1 and GLUT-14 is of high prognostic value. It gives additional information to UICC stages and identifies patients with inferior prognosis. If confirmed in prospective studies, these markers need to be considered for future classification systems.


Similar content being viewed by others
References
Kwee RM, Kwee TC. Imaging in local staging of gastric cancer: a systematic review. J Clin Oncol. 2007;25:2107–16.
Siewert JR, Bottcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449–61.
Kim JP, Kwon OJ, Oh ST, Yang HK. Results of surgery on 6589 gastric cancer patients and immunochemosurgery as the best treatment of advanced gastric cancer. Ann Surg. 1992;216:269–78 (discussion 278–269).
Hansson LE, Sparen P, Nyren O. Survival in stomach cancer is improving: results of a nationwide population-based Swedish study. Ann Surg. 1999;230:162–9.
Yasui W, Oue N, Aung PP, Matsumura S, Shutoh M, Nakayama H. Molecular-pathological prognostic factors of gastric cancer: a review. Gastric Cancer. 2005;8:86–94.
Alakus H, Holscher AH, Grass G, et al. Extracapsular lymph node spread: a new prognostic factor in gastric cancer. Cancer. 2010;116:309–15.
Alakus H, Grass G, Hennecken JK, et al. Clinicopathological significance of MMP-2 and its specific inhibitor TIMP-2 in gastric cancer. Histol Histopathol. 2008;23:917–23.
Berlth F, Bollschweiler E, Drebber U, Hoelscher AH, Moenig S. Pathohistological classification systems in gastric cancer: diagnostic relevance and prognostic value. WJG World J Gastroenterol. 2014;20:5679–84.
Barrett MP, Walmsley AR, Gould GW. Structure and function of facilitative sugar transporters. Curr Opin Cell Biol. 1999;11:496–502.
Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34:121–38.
Montel-Hagen A, Kinet S, Manel N, et al. Erythrocyte GLU-1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell. 2008;132:1039–48.
Mueckler M. Facilitative glucose transporters. Eur J Biochem. 1994;219:713–25.
Birnbaum MJ, Haspel HC, Rosen OM. Transformation of rat fibroblasts by FSV rapidly increases glucose transporter gene transcription. Science. 1987;235:1495–8.
Kim WS, Kim YY, Jang SJ, Kimm K, Jung MH. Glucose transporter 1 (GLUT1) expression is associated with intestinal type of gastric carcinoma. J Korean Med Sci. 2000;15:420–4.
Kawamura T, Kusakabe T, Sugino T, et al. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer. 2001;92:634–41.
Wu X, Freeze HH. GLUT14, a duplicon of GLUT3, is specifically expressed in testis as alternative splice forms. Genomics. 2002;80:553–7.
The Human Protein Atlas. Retrieved 2 February 2015. http://www.proteinatlas.org/ENSG00000173262-SLC2A14/cancer.
Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32.
Griffiths EA, Pritchard SA, Welch IM, Price PM, West CM. Is the hypoxia-inducible factor pathway important in gastric cancer? Eur J Cancer. 2005;41:2792–805.
Urano N, Fujiwara Y, Doki Y, et al. Overexpression of hypoxia-inducible factor-1 alpha in gastric adenocarcinoma. Gastric Cancer. 2006;9:44–9.
Jung JH, Im S, Jung ES, Kang CS. Clinicopathological implications of the expression of hypoxia-related proteins in gastric cancer. Int J Med Sci. 2013;10:1217–23.
Knight G, Earle CC, Cosby R, et al. Neoadjuvant or adjuvant therapy for resectable gastric cancer: a systematic review and practice guideline for North America. Gastric Cancer. 2013;16:28–40.
A Japanese Gastric Cancer. Japanese classification of gastric carcinoma. 2nd English ed. Gastric Cancer. 1998;1:10–24.
Catalano V, Labianca R, Beretta GD, Gatta G, de Braud F, Van Cutsem E. Critical reviews in oncology/hematology. Gastric Cancer. 2009;71:127–64.
Marin-Hernandez A, Gallardo-Perez JC, Ralph SJ, Rodriguez-Enriquez S, Moreno-Sanchez R. HIF-1alpha modulates energy metabolism in cancer cells by inducing overexpression of specific glycolytic isoforms. Mini Rev Med Chem. 2009;9:1084–101.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33.
Yamamoto T, Seino Y, Fukumoto H, et al. Overexpression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun. 1990;170:223–30.
Wang BY, Kalir T, Sabo E, Sherman DE, Cohen C, Burstein DE. Immunohistochemical staining of GLUT1 in benign, hyperplastic, and malignant endometrial epithelia. Cancer. 2000;88:2774–81.
Rudlowski C, Moser M, Becker AJ, et al. GLUT1 mRNA and protein expression in ovarian borderline tumors and cancer. Oncology. 2004;66:404–10.
Rudlowski C, Becker AJ, Schroder W, Rath W, Buttner R, Moser M. GLUT1 messenger RNA and protein induction relates to the malignant transformation of cervical cancer. Am J Clin Pathol. 2003;120:691–8.
Ogawa J, Inoue H, Koide S. Glucose-transporter-type-I-gene amplification correlates with sialyl-Lewis-X synthesis and proliferation in lung cancer. Int J Cancer. 1997;74:189–92.
Nishioka T, Oda Y, Seino Y, et al. Distribution of the glucose transporters in human brain tumors. Cancer Res. 1992;52:3972–9.
Nagase Y, Takata K, Moriyama N, Aso Y, Murakami T, Hirano H. Immunohistochemical localization of glucose transporters in human renal cell carcinoma. J Urol. 1995;153(3 Pt 1):798–801.
Haber RS, Rathan A, Weiser KR, et al. GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer. 1998;83:34–40.
Cantuaria G, Fagotti A, Ferrandina G, et al. GLUT-1 expression in ovarian carcinoma: association with survival and response to chemotherapy. Cancer. 2001;92:1144–50.
Brown RS, Wahl RL. Overexpression of GLUT-1 glucose transporter in human breast cancer: an immunohistochemical study. Cancer. 1993;72:2979–85.
Yamada A, Oguchi K, Fukushima M, Imai Y, Kadoya M. Evaluation of 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography in gastric carcinoma: relation to histological subtypes, depth of tumor invasion, and glucose transporter-1 expression. Ann Nucl Med. 2006;20:597–604.
Yip CH, Rhodes A. Estrogen and progesterone receptors in breast cancer. Future Oncol. 2014;10:2293–301.
Abouzeid AH, Patel NR, Rachman IM, Senn S, Torchilin VP. Anti-cancer activity of anti-GLUT1 antibody-targeted polymeric micelles co-loaded with curcumin and doxorubicin. J Drug Target. 2013;21:994–1000.
Acknowledgment
This study did not receive financial or other support from any organizations or other people not listed as authors.
Conflict of interest
There are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berlth, F., Mönig, S., Pinther, B. et al. Both GLUT-1 and GLUT-14 are Independent Prognostic Factors in Gastric Adenocarcinoma. Ann Surg Oncol 22 (Suppl 3), 822–831 (2015). https://doi.org/10.1245/s10434-015-4730-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-015-4730-x