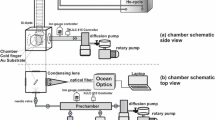
Abstract
A simple device based on splat cooling to liquid nitrogen temperatures is presented. Its application to the amorphization of binary aqueous solutions by fast cooling is demonstrated. The fraction of amorphous material obtained is 90% in eutectic solutions. Diffraction patterns of the vitrified solutions are presented and discussed.
Similar content being viewed by others
References
C.A. Angell, E.J. Sare, J. Chem. Phys. 52, 1058 (1970)
C. Angell, E. Sare, Cryo-letters 1, 257 (1980)
O. Mishima, J. Chem. Phys. 123, 154506 (2005)
C.J. Tainter, L. Shi, J.L. Skinner, J. Chem. Phys. 140, 134503 (2014)
G. Vuillard, Ann. Chim (Paris) 2, 233 (1957)
A. Elarby-Aouizerat, J.F. Jal, C. Ferradou, J. Dupuy, P. Chieux, A. Wright, J. Phys. Chem. 87, 4170 (1983)
M. Gordon, J.S. Taylor, J. Appl. Chem. 2, 493 (1952)
W. Brostow, R. Chiu, I.M. Kalogeras, A. Vassilikou-Dova, Mat. Lett. 62, 3152 (2008)
M. Warkentin, J.P. Sethna, R.E. Thorne, Phys. Rev. Lett. 110, 015703 (2013)
V. Berejnov, N.S. Husseini, O.A. Alsaied, R.E. Thorne, J. Appl. Crystallogr. 39, 244 (2006)
M. Allix, in Du verre au cristal: Nucléation, croissance et démixtion, de la recherche aux applications, edited by D.R. Neuville, L. Cormier, D. Caurant (EDP Sciences, Les Ulis, 2013), Chap. 17
C.A. Angell, Science 319, 582 (2008)
V. Velikov, S. Borick, C.A. Angell, Science 294, 2335 (2001)
T. Loerting, K. Winkel, M. Seidl, M. Bauer, C. Mitterdorfer, P.H. Handle, C.G. Salzmann, E. Mayer, J.L. Finney, D.T. Bowron, Phys. Chem. Chem. Phys. 13, 8783 (2011)
P. Ball, H2O: A Biography of Water (Hachette, UK, 1999)
P. Jenniskens, D. Blake, ApJ 473, 1104 (1996)
K. Lodders, ApJ 591, 1220 (2003)
K. Wada, H. Tanaka, T. Suyama, H. Kimura, T. Yamamoto, ApJ 677, 1296 (2008)
B. Gundlach, Y. Skorov, J. Blum, Icarus 213, 710 (2011)
B. Gundlach, S. Kilias, E. Beitz, J. Blum, Icarus 214, 717 (2011)
K. Ros, A. Johansen, Astr. & Astrophys. 552, A137 (2013)
T. Koop, Proceedings of the International School of Physics Enrico Fermi (Water: Fundamentals as the Basis for Understanding the Environment and Promoting Technology) 187, 45 (2015)
E. Mayer, J. Appl. Phys. 58, 663 (1985)
E. Burton, W. Oliver, Nature 135, 505 (1935)
M. Nič, J. Jirát, B. Košata, A. Jenkins, A. McNaught, eds., IUPAC Compendium of Chemical Terminology (IUPAC, 2009)
O. Mishima, L.D. Calvert, E. Whalley, Nature 310, 393 (1984)
J.S. Tse, D.D. Klug, C.A. Tulk, I. Swainson, E.C. Svensson, C.K. Loong, V. Shpakov, V.R. Belosludov, R.V. Belosludov, Y. Kawazoe, Nature 400, 647 (1999)
R.J. Nelmes, J.S. Loveday, T. Strässle, C.L. Bull, M. Guthrie, G. Hamel, S. Klotz, Nat. Phys. 2, 414 (2006)
K. Winkel, E. Mayer, T. Loerting, J. Phys. Chem. B 115, 14141 (2011)
G.N. Ruiz, L.E. Bove, H.R. Corti, T. Loerting, Phys. Chem. Chem. Phys. 16, 18553 (2014)
P. Brüggeller, E. Mayer, Nature 288, 569 (1980)
O. Mishima, J. Phys. Chem. B 115, 14064 (2011)
H. Jones, Rep. Prog. Phys. 36, 1425 (1973)
R.C. Ruhl, Mater. Sci. Eng. 1, 313 (1967)
T.C. Hansen, P.F. Henry, H.E. Fischer, J. Torregrossa, P. Convert, Meas. Sci. Technol. 19, 034001 (2008)
A.-A. Ludl, L.E. Bove, A.M. Saitta, M. Salanne, T. Hansen, C.L. Bull, R. Gaal, S. Klotz, Phys. Chem. Chem. Phys. 17, 14054 (2015)
J. Rodríguez-Carvajal, Physica B 192, 55 (1993)
T. Roisnel, J. Rodríguez-Carvajal, WinPLOTR: a windows tool for powder diffraction pattern analysis, in, Materials Science Forum (Transtec Publications; 1999, 2001), Vol. 378, p. 118
W.F. Kuhs, M.S. Lehmann, Water Sci. Rev. 2, 1 (1986)
B. Klewe, B. Pedersen, Acta Crystallogr., Sect. B: Crystallogr. Crystal. Chem. 30, 2363 (1974)
K. Muldrew, L.E. McGann, Ch. 6 phase diagrams (1997), http://people.ucalgary.ca/kmuldrew/cryo_course/cryo_chap6_1.html
H. Landolt, R. Börnstein, H. Borchers, W. Helling, E. Schmidt, Zahlenwerte und Funktionen aus Physik, Chemie, Astronomie, Geophysik und Technik: Technik. 2. Teil. [Stoffwerte und Verhalten von metallischen Werkstoffen.] Bandteil b. Sinterwerkstoffe. Schwermetalle (ohne Sonderwerkstoffe) (Springer-Verlag, 1964)
T. Williams, C. Kelley et al., Gnuplot 4.6: an interactive plotting program, http://gnuplot.sourceforge.net/ (2014)
A.A. Ludl, High pressure salty ice – exploring the phase diagram of electrolyte solutions in extreme conditions (2015)
L.E. Bove, S. Klotz, J. Philippe, A. Saitta, Phys. Rev. Lett. 106, 125701 (2011)
L.E. Bove, C. Dreyfus, A. Polian, B. Bonello, I. Malfanti, A. Taschin, R. Torre, R.M. Pick, J. Chem. Phys. 134, 034514 (2011)
A.-A. Ludl, L.E. Bove, D. Corradini, A.M. Saitta, M. Salanne, C.L. Bull, S. Klotz, Phys. Chem. Chem. Phys. 19, 1875 (2017)
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ludl, AA., Bove, L.E., Li, J. et al. Quenching device for electrolytic aqueous solutions. Eur. Phys. J. Spec. Top. 226, 1051–1063 (2017). https://doi.org/10.1140/epjst/e2016-60244-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1140/epjst/e2016-60244-8