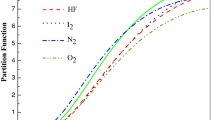
Abstract
In this work, we have studied such thermal function of diatomic molecules like hydrogen dimer, carbon monoxide, nitrogen dimer and lithium hydride using improved deformed exponential-type potential (IDEP). To this end, the energy spectra of the IDEP are obtained applying Greene-Aldrich approximation and appropriate coordinate transformation within the framework of non-relativistic quantum mechanics. With calculated energy eigenstates, we have deduced the partition function and such thermodynamic functions like specific heat in constant pressure, enthalpy and Gibbs free energy by employing the Poisson summation formula. We have compared our results with experimental data, and there is a good agreement between them.



Similar content being viewed by others
References
X.-L. Peng, R. Jiang, C.-S. Jia, L.-H. Zhang, Y.-L. Zhao, Gibbs free energy of gaseous phosphorus dimer. Chem. Eng. Sci. 190, 122–125 (2018)
C.-S. Jia, Y.-F. Diao, X.-J. Liu, P.-Q. Wang, J.-Y. Liu, G.-D. Zhang, Equivalence of the Wei potential model and Tietz potential model for diatomic molecules. J. Chem. Phys. 137, 014101 (2012)
R. Khordad, A. Ghanbari, Theoretical prediction of thermodynamic functions of TiC: Morse ring-shaped potential. J. Low Temp. Phys. 199, 1198–1210 (2020)
A. Ghanbari, R. Khordad, Theoretical prediction of thermodynamic properties of N2 and CO using pseudo harmonic and Mie-type potentials. Chem. Phys. 534, 110732 (2020)
R. Khordad, A. Avazpour, A. Ghanbari, Exact analytical calculations of thermodynamic functions of gaseous substances. Chem. Phys. 517, 30–35 (2019)
C.-S. Jia, C.-W. Wang, L.-H. Zhang, X.-L. Peng, R. Zeng, X.-T. You, Partition function of improved Tietz oscillators. Chem. Phys. Lett. 676, 150–153 (2017)
C.-S. Jia, C.-W. Wang, L.-H. Zhang, X.-L. Peng, H.-M. Tang, R. Zeng, Enthalpy of gaseous phosphorus dimer. Chem. Eng. Sci. 183, 26–29 (2018)
C.-S. Jia, C.-W. Wang, L.-H. Zhang, X.-L. Peng, H.-M. Tang, J.-Y. Liu, Y. Xiong, R. Zeng, Predictions of entropy for diatomic molecules and gaseous substances. Chem. Phys. Lett. 692, 57–60 (2018)
R. Khordad, A. Ghanbari, Analytical calculations of thermodynamic functions of lithium dimer using modified Tietz and Badawi-Bessis-Bessis potentials. Comput. Theor. Chem. 1155, 1–8 (2019)
M. Kria, K. Feddi, N. Aghoutane, M. El-Yadri, L. Pérez, D. Laroze, F. Dujardin, E. Feddi, Thermodynamic properties of SnO2/GaAs core/sell nanofiber. Physica A 560, 125104 (2020)
L. Guo, J. Du, Thermodynamic potentials and thermodynamic relations in nonextensive thermodynamics. Physica A 390, 183–188 (2011)
P. Ammendola, F. Raganati, R. Chirone, CO2 adsorption on a fine activated carbon in a sound assisted fluidized bed: thermodynamics and kinetics. Chem. Eng. J. 322, 302–313 (2017)
J. Bedia, C. Belver, S. Ponce, J. Rodriguez, J. Rodriguez, Adsorption of antipyrine by activated carbons from FeCl3-activation of Tara gum. Chem. Eng. J. 333, 58–65 (2018)
S. Dastidar, C.J. Hawley, A.D. Dillon, A.D. Gutierrez-Perez, J.E. Spanier, A.T. Fafarman, Quantitative phase-change thermodynamics and metastability of perovskite-phase cesium lead iodide. J. Phys. Chem. Lett. 8, 1278–1282 (2017)
V.L. Zherebtsov, M.M. Peganova, Water solubility versus temperature in jet aviation fuel. Fuel 102, 831–834 (2012)
Y.I. Prylutskyy, A. Shut, M. Miroshnychenko, A. Suprun, Thermodynamic and mechanical properties of skeletal muscle contraction. Int. J. Thermophys. 26, 827–835 (2005)
N.P. Stadie, M. Murialdo, C.C. Ahn, B. Fultz, Anomalous isosteric enthalpy of adsorption of methane on zeolite-templated carbon. J. Am. Chem. Soc. 135, 990–993 (2013)
S.A. Moses, J.P. Covey, M.T. Miecnikowski, B. Yan, B. Gadway, J. Ye, D.S. Jin, Creation of a low-entropy quantum gas of polar molecules in an optical lattice. Science 350, 659–662 (2015)
R. Mebsout, S. Amari, S. Méçabih, B. Abbar, B. Bouhafs, Spin-polarized calculations of magnetic and thermodynamic properties of the full-heusler CO2 MnZ (Z= Al, Ga). Int. J. Thermophys. 34, 507–520 (2013)
T. Ibrahim, K. Oyewumi, S. Wyngaardt, Analytical solution of N-dimensional Klein–Gordon and Dirac equations with Rosen–Morse potential. Eur. Phys. J. Plus 127, 100 (2012)
X.-Y. Gu, S.-H. Dong, Energy spectrum of the Manning-Rosen potential including centrifugal term solved by exact and proper quantization rules. J. Math. Chem. 49, 2053 (2011)
T. Tietz, A new potential energy function for diatomic molecules. J. Phys. Soc. Jpn. 18, 1647–1649 (1963)
Y.P. Varshni, Comparative study of potential energy functions for diatomic molecules. Rev. Mod. Phys. 29, 664 (1957)
X.-J. Xie, C.-S. Jia, Solutions of the Klein-Gordon equation with the Morse potential energy model in higher spatial dimensions. Phys. Scr. 90, 035207 (2015)
H. Baltache, R. Khenata, M. Sahnoun, M. Driz, B. Abbar, B. Bouhafs, Full potential calculation of structural, electronic and elastic properties of alkaline earth oxides MgO, CaO and SrO. Physica B 344, 334–342 (2004)
M.M. Nieto, L. Simmons Jr., V.P. Gutschick, Coherent states for general potentials. VI. Conclusions about the classical motion and the WKB approximation. Phys. Rev. D 23, 927 (1981)
H. Louis, B.I. Ita, N.I. Nzeata, Approximate solution of the Schrödinger equation with Manning–Rosen plus Hellmann potential and its thermodynamic properties using the proper quantization rule. Eur. Phys. J. Plus 134, 315 (2019)
M. Oliveira, A.G. Schmidt, Exact solutions of Schrödinger and Pauli equations on a spherical surface and interacting with Pöschl–Teller double-ring-shaped potential. Physica E 120, 114029 (2020)
M. Udoh, U. Okorie, M. Ngwueke, E. Ituen, A. Ikot, Rotation-vibrational energies for some diatomic molecules with improved Rosen–Morse potential in D-dimensions. J. Mol. Model. 25, 170 (2019)
V. Badalov, B. Baris, K. Uzun, Bound states of the D-dimensional Schrödinger equation for the generalized Woods–Saxon potential. Mod. Phys. Lett. A 34, 1950107 (2019)
C.-S. Jia, Y. Li, Y. Sun, J.-Y. Liu, L.-T. Sun, Bound states of the five-parameter exponential-type potential model. Phys. Lett. A 311, 115–125 (2003)
H. Bakhti, A. Diaf, M. Hachama, Computing thermodynamic properties of the O2 and H2 molecules with multi-parameter exponential-type potential. Comput. Theor. Chem. 1185, 112879 (2020)
E. Eyube, U. Wadata, S. Najoji, Energy eigenvalues and eigenfunctions of a diatomic molecule in quadratic exponential-type potential. Niger. Ann. Pure Appl. Sci. 3, 240–251 (2020)
K.-X. Fu, M. Wang, C.-S. Jia, Improved five-parameter exponential-type potential energy model for diatomic molecules. Commun. Theor. Phys. 71, 103 (2019)
B.J. Xie, C.S. Jia, Improved multiparameter exponential-type potential for diatomic molecules. Int. J. Quantum Chem. 120, e26058 (2020)
P. Linstorm, Nist chemistry webbook, nist standard reference database number 69. J. Phys. Chem. Ref. Data Monograph 9, 1–1951 (1998)
C. Onate, M. Onyeaju, A. Ikot, O. Ebomwonyi, Eigen solutions and entropic system for Hellmann potential in the presence of the Schrödinger equation. Eur. Phys. J. Plus 132, 462 (2017)
C.-S. Jia, T. Chen, L.-G. Cui, Approximate analytical solutions of the Dirac equation with the generalized Pöschl–Teller potential including the pseudo-centrifugal term. Phys. Lett. A 373, 1621–1626 (2009)
J.A. Kunc, F.J. Gordillo-Vazquez, Rotational−vibrational levels of diatomic molecules represented by the Tietz−Hua rotating oscillator. J. Phys. Chem. A 101, 1595–1602 (1997)
M. Rafi, R. Al-Tuwirqi, H. Farhan, I. Khan, A new four-parameter empirical potential energy function for diatomic molecules. Pramana 68, 959–965 (2007)
C.-S. Jia, L.-H. Zhang, C.-W. Wang, Thermodynamic properties for the lithium dimer. Chem. Phys. Lett. 667, 211–215 (2017)
M. Deng, C.-S. Jia, Prediction of enthalpy for nitrogen gas. Eur. Phys. J. Plus 133, 258 (2018)
C.-S. Jia, R. Zeng, X.-L. Peng, L.-H. Zhang, Y.-L. Zhao, Entropy of gaseous phosphorus dimer. Chem. Eng. Sci. 190, 1–4 (2018)
C.-S. Jia, L.-H. Zhang, X.-L. Peng, J.-X. Luo, Y.-L. Zhao, J.-Y. Liu, J.-J. Guo, L.-D. Tang, Prediction of entropy and Gibbs free energy for nitrogen. Chem. Eng. Sci. 202, 70–74 (2019)
M. Farout, A. Bassalat, S.M. Ikhdair, Feinberg–Horodocki exact momentum states of improved deformed exponential-type potential. arXiv: 2007.14789 (2020)
U.S. Okorie, A.N. Ikot, E.O. Chukwuocha, The improved deformed exponential-type potential energy model for N2, NI, ScI, and RbH diatomic molecules. Bull. Korean Chem. Soc. 41, 609–614 (2020)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Habibinejad, M., Ghanbari, A. Enthalpy, Gibbs free energy and specific heat in constant pressure for diatomic molecules using improved deformed exponential-type potential (IDEP). Eur. Phys. J. Plus 136, 400 (2021). https://doi.org/10.1140/epjp/s13360-021-01338-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/s13360-021-01338-7