Abstract
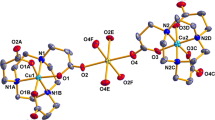
A new Cu(II) based metal-organic framework (MOF) having formula [Cu3(H2Tar)(Tar)-(H2O)(Bpa)] · 3H2O (I) (H4Tar = tartaric acid, Bpa = 1,2-bis(4-pyridyl)ethane) has been characterized by elemental analysis, FT-IR spectra, thermal analysis, and single-crystal X-ray diffraction (CIF file CCDC no. 1575136). In I, the Cu(II) center are connected by four symmetry-related tartrate ligands into a 2D layer encapsulating trinuclear cluster, which are further bridged by Bpa molecules into a 3D framework. Complex I exhibits strong ferromagnetic behavior between metal centers.
Similar content being viewed by others
References
Kahn, O., Molecular Magnetism, New York: VCH, 1993.
Braga, D., Grepioni, F., and Desiraju, G.R., Chem. Rev., 1998, vol. 98, p. 1375.
Ferrer, S., Haasnoot, J.G., Reedijk, J., et al., Inorg. Chem., 2000, vol. 39, p. 1859.
Liu, J.C., Guo, G.C., Huang, J.S., et al., Inorg. Chem., 2003, vol. 42, p. 235.
Cage, B., Cotton, F.A., Dalal, N.S., et al., J. Am. Chem. Soc., 2003, vol. 125, p. 5270.
Gojon, E., Gaillard, J., Latour, J.M., et al., Inorg. Chem., 1987, vol. 26, p. 2047.
Murphy, B.P., Coord. Chem. Rev., 1993, vol. 124, p. 63.
Haasnoot, J.G., Coord. Chem. Rev., 2000, vol. 200, p. 131.
Bian, H.D., Xu, J.Y., Gu, W., et al., Polyhedron, 2003, vol. 22, p. 2927.
Doyle, R.P., Julve, M., Lloret, F., et al., Dalton Trans., 2006, p. 2081.
Miller, J.S., Dalton Trans., 2006, p. 2742.
Chui, S.S.Y., Lo, S.M.F., Charmant, J.P.H., et al., Science, 1999, vol. 283, p. 1148.
Konar, S., Zangrando, E., Drew, M.G.B., et al., Dalton Trans., 2004, p. 260.
Konar, S., Mukherjee, P.S., Zangrando, E., et al., Angew. Chem. Int. Ed., 2002, vol. 41, p. 1561.
Xiang, S.C., Wu, X.T., Zhang, J.J., et al., J. Am. Chem. Soc., 2005, vol. 127, p. 16352.
Murrie, M., Teat, S.J., Stoecki-Evans, H., et al., Angew. Chem. Int. Ed., 2003, vol. 42, p. 4653.
Thushari, S., Cha, J.A.K., Sung, H.H.-Y., et al., Chem. Commun., 2005, p. 5515.
Zhang, X.M., Hou, J.J., and Wu, H.S., Dalton Trans., 2004, p. 3437.
Julve, M., Verdaguer, M., Kahn, O., et al., Inorg. Chem., 1983, vol. 22, p. 368.
Carranza, J., Grove, H., Sletten, J., et al., Eur. J. Inorg. Chem., 2004, p. 4836.
Julve, M., Verdaguer, M., Gleizes, A., et al., Inorg. Chem., 1984, vol. 23, p. 3808.
Gleizes, A., Julve, M., Verdaguer, M., et al., J. Chem. Soc., Dalton Trans. (1972–1999), 1992, p. 3209.
Zhang, X.-M., Coord. Chem. Rev., 2005, vol. 249, p. 1201.
Hennigar, T.L., MacQuarrie, D.C., Losier, P., et al., Angew. Chem. Int. Ed., 1997, vol. 36, p. 972.
Zhou, Y.X., Shen, X.Q., Liu, H.L., et al., Syn. React. Inorg. Met., 2006, vol. 36, p. 563.
Sheldrick, G.M., SHELXL-97, Program for Structure Determination and Refinement, Göttingen: Univ. of Göttingen, 1997.
Duan, L.M., Xie, F., Chen, X.Y., et al., Cryst. Growth Des., 2006, vol. 6, p. 1101.
Butcher, R.J. and O’Connor, C.J.E., Sinn. Inorg. Chem., 1981, vol. 20, p. 537.
Bailey, N.A., Fenton, D.E., Moddy, R., et al., J. Chem. Soc., Dalton Trans. (1972–1999), 1988, p. 2817.
Angaroni, A., Ardizzoiz, G.A., Beringhelli, T., et al., Dalton Trans., 1990, p. 2817.
Suh, M.P., Han, M.Y., Lee, J.H., et al., J. Am. Chem. Soc., 1998, vol. 120, p. 3819.
Tong, M.L., Wu, Y.M., Tong, Y.X., et al., Eur. J. Inorg. Chem., 2003, p. 2385.
Lin, J.G., Zang, S.Q., Tian, Z.F., et al., CrystEng-Comm, 2007, vol. 9, p. 915.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Zhou, E.H., Li, B.H., Zhong, H.R. et al. Ferromagnetic Behavior of an Uncommon Trinuclearcopper(II) Coordination Polymer Based on Tartarate and 1,2-bis(4-Pyridyl)ethane Linker. Russ J Coord Chem 44, 432–438 (2018). https://doi.org/10.1134/S1070328418070072
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328418070072