Abstract
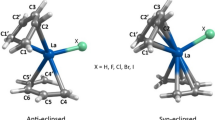
The main goal of this research is to investigate the structural and thermochemical aspects of complexation between La3+ with tetrapropyl malonamide (TPMA) and tetrapropyl diglycolamide (TPDGA) ligands via density functional theory (DFT) methods. In this respect, the structural parameters of [La-TPMA]3+ and [La-TPDGA]3+ complexes have been calculated and compared with the available X-ray crystallographic data. These comparisons revealed that both calculated structural values using B3LYP and M06 are in a reliable agreement with X-ray crystal structure with a near accuracy. In the next step, the more efficiency of diglycolamides in comparison with malonamides in the extraction of La3+ have been analyzed by calculating thermochemical properties of the complexation. It should be stated that this issue has been observed in many experimental elucidations. In the next step, the inclusion of solvent effects on thermodynamical properties of complexation has been evaluated via polarized continuum model (PCM) calculations. In this context, enthalpy and Gibbs free energy changes have been determined in the presence of three solvents, chloroform, toluene and n-hexane. Our obtained results demonstrate that using n-hexane as solvent is more favorable thermodynamically than chloroform and toluene that confirms the previously observed experiments. Finally, the bond orders of some selected key bonds in TPMA and TPDGA ligands and their corresponded La3+ complexes have been evaluated comparatively to analyze the electronic features of coordination in [La-TPMA]3+ and [La-TPDGA]3+ complexes.
Similar content being viewed by others
References
S. Bourg, C. Hill, C. Caravaca, C. Rhodes, C. Ekberg, R. Taylor, A. Geist, G. Modolo, L. Cassayre, R. Malmbeck, M. Harrison, G. Angelis, A. Espartero, S. Bouvet, and N. Ouvrier, Nucl. Eng. Des. 241, 3427 (2001).
K. L. Nash, C. Madic, J. N. Mathur, J. Lacquement, in Chemistry of the Actinide and Transactinide Elements, Ed. by L. R. Morss, N. M. Edelstein, J. Fuger, and J. J. Katz, 3rd ed. (Springer, Netherlands, 2006).
Y. Morita, J. P. Glatz, M. Kubota, L. Koch, G. Pagliosa, and K. Roemer, Solvent Extract. Ion Exchange 14, 385 (1996).
R. Malmbeck, O. Courson, G. Pagliosa, K. Romer, B. Satmark, J. P. Glatz, and P. Baron, Radiochim. Acta 88, 856 (2008).
Y. Wen, Z. Qin, and W. Liu, J. Radioanal. Nucl. Chem. 250, 285 (2001).
Y. Sasaki, Y. Sugo, S. Suzuki, and S. Tachimori, Solvent Extr. Ion Exch. 19, 91 (2001).
H. Stephan, K. Gloe, J. Beger, and P. Muhl, Solvent Extract. Ion Exchange 9, 435 (1991).
H. Stephan, K. Gloe, J. Beger, and P. Muhl, Solvent Extract. Ion Exchange 9, 459 (1991).
E. A. Mowafy and H. F. Aly, Solvent Extract. Ion Exchange 20, 177 (2002).
C. Musikas and H. Hubert, Solvent Extract. Ion Exchange 5, 877 (1987).
C. Cuillerdier, C. Musikas, P. Hoel, N. L. Igond, and X. Vitart, Separat. Sci. Technol. 26, 1229 (1991).
J. Yao, R. M. Wharf, and G. R. Choppin, in Separation of Elements, Ed. by K. L. Nash and G. R. Choppin (Plenum Press, New York, 1995).
Y. Sasaki and G. R. Choppin, J. Radioanal. Nucl. Chem. 246, 267 (1997).
Y. Sasaki and G. R. Choppin, Radiochim. Acta 180, 85 (1998).
H. Narita, T. Yaita, K. Tamura, and S. Tachimori, Radiochim. Acta 81, 223 (1998).
H. Narita and S. Tachimori, J. Radioanal. Nucl. Chem. 239, 381 (1999).
Y. Sasaki, Y. Sugo, S. Suzuki, and S. Tachimori, Solvent. Extract. Ion Exchange 19, 91 (2001).
Y. Sasaki, P. Rapold, M. Arisaka, M. Hirata, T. Kimura, C. Hill, and G. Cote, Solvent Extract. Ion Exchange 25, 187 (2007).
E. P. Horwitz, K. A. Martin, and H. Diamond, Solvent Extract. Ion Exchange 6, 859 (1988).
M. Hirata, P. Guilbaud, M. Dobler, and S. Tachimori, Phys. Chem. Chem. Phys. 5, 691 (2003).
R. G. Pearson, J. Chem. Educ. 64, 561 (1987).
A. D. Becke, J. Chem. Phys. 98, 1372 (1993).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B 37, 785 (1988).
S. Kannan, M. A. Moody, C. L. Barnes, and P. B. Duval, Inorg. Chem. 47, 4691 (2008).
S. A. El-Reefy, E. A. Mowafy, M. M. Abdel-Badei, and H. F. Aly, Radiochim. Acta 77, 195 (1997).
L. Spjuth, J. O. Liljenzin, M. I. Hudson, M. G. B. Drew, P. B. Iveson, and C. Madic, Solvent Extract. Ion Exchange 18, 1 (2000).
L. Nigond, C. Musikas, and C. Cuillerdier, Solvent Extract. Ion Exchange 12, 297 (1994).
V. Barone and M. Cossi, J. Phys. Chem. A 102, 1995 (1998).
A. D. Becke, J. Chem. Phys. 98, 5648 (1993).
D. G. Truhlar and Y. Zhao, Theor. Chem. Account. 120, 215 (2008).
M. Dolg, H. Stoll, A. Savin, and H. Preuss, Theor. Chim. Acta 75, 173 (1989).
M. Dolg, H. Stoll, and H. Preuss, Theor. Chim. Acta 85, 441 (1993).
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. J. Su, T. L. Windus, M. Dupuis, and J. A. Montgomery, J. Comput. Chem. 14, 1347 (1993).
A. D. Becke, Phys. Rev. A 38, 3098 (1988).
S. H. Vosko, L. Wilk, and M. Nusair, Can. J. Phys. 58, 1200 (1980).
G. A. Shamov and G. Schreckenbach, J. Phys. Chem. A 109, 10961 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Hosseinnejad, T., Dehghanpour, S. & Basiri-nasab, S. A DFT study on the complexation of La3+ ion with malonamide and diglycolamide ligands. Russ. J. Phys. Chem. 88, 2004–2011 (2014). https://doi.org/10.1134/S0036024414110156
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024414110156