Abstract
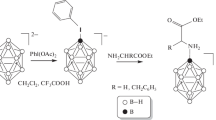
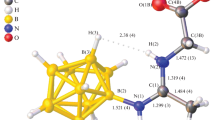
A novel scheme of multi-step synthesis for N-borylated dipeptide R-GlyPheOEt is proposed, which is based on the nucleophilic addition of amino acid derivatives to the [2-B10H9NCCH3]– anion. The products obtained at each step were studied by NMR, IR spectroscopy, and ESI mass spectrometry. The single-crystal structure of (NBu4)[2-B10H9NH=C(NH2CH2COOtC4H9)CH3] was identified by XRD.


Similar content being viewed by others
REFERENCES
V. Bregadze and I. Sivaev, Boron Sci. (CRC, 2011). https://doi.org/10.1201/b11199-14
H. R. Snyder, A. J. Reedy, W. J. Lennarz, et al., J. Am. Chem. Soc. 80, 835 (1958). https://doi.org/10.1021/ja01537a021
S. Diaz, A. Gonzalez, R. S. de Gonzalez, et al., J. Organomet. Chem. 610, 25 (2000). https://doi.org/10.1016/S0022-328X0000363-6
R. R. Srivastava, R. R. Singhaus, and G. W. Kabalka, Org. Chem. 64, 8495 (1999). https://doi.org/10.1021/jo990878c
G. W. Kabalka, M.-L. Yao, and Z. Wu, Org. Process Res. Dev. 10, 1059 (2006). https://doi.org/10.1021/op060052u
P. Ryynänen, A. Kangasmäki, P. Hiismaki, et al., Phys. Med. Biol. 47 (4), 737 (2002). https://doi.org/10.1088/0031-9155/47/5/304
P. F. Kaiser, Q. I. Churches, and C. A. Hutton, Aust. J. Chem. 60, 799 (2007). https://doi.org/10.1071/CH07103
G. W. Kabalka and M. L. Yao, Anticancer. Agents Med. Chem. 6, 111 (2006). https://doi.org/10.2174/187152006776119144
O. Leukart, M. Caviezel, A. Eberle, et al., Helv. Chim. Acta 59, 2184 (1976). https://doi.org/10.1002/hlca.19760590630
R. R. Srivastava, R. R. Singhaus, and G. W. Kabalka, Org. Chem. 62, 4476 (1997). https://doi.org/10.1021/jo970148+
S. B. Kahl and R. A. Kasar, J. Am. Chem. Soc. 118, 1223 (2002). https://doi.org/10.1021/ja9534260
T. He and R. A. Musah, ACS Omega 4, 3820 (2019). https://doi.org/10.1021/acsomega.8b03407
P. Perugini and F. Pavanetto, J. Microencapsul. 15, 473 (1998). https://doi.org/10.3109/02652049809006874
F. Pavanetto and P. Perugini, Drug Deliv. 7, 97 (2002). https://doi.org/10.1080/107175400266669
S. Martini, S. Ristori, A. Pucci, et al., Biophys. Chem. 111, 27 (2004). https://doi.org/10.1016/j.bpc.2004.03.010
V. Bregadze, A. Semioshkin, and I. Sivaev, Appl. Radiat. Isot. 69, 1774 (2011). https://doi.org/10.1016/j.apradiso.2011.01.043
S. Kusaka, Y. Hattori, K. Uehara, et al., Appl. Radiat. Isot. 69, 1768 (2011). https://doi.org/10.1016/j.apradiso.2011.03.042
Y. Hattori, S. Kusaka, M. Mukumoto, et al., J. Med. Chem. 55, 6980 (2012). https://doi.org/10.1021/jm300749q
K. Y. Zhizhin, A. P. Zhdanov, and N. T. Kuznetsov, Russ. J. Inorg. Chem. 55, 2089 (2010). https://doi.org/10.1134/S0036023610140019
I. N. Klyukin, A. P. Zhdanov, G. A. Razgonyaeva, et al., Russ. J. Inorg. Chem. 58, 1395 (2013). https://doi.org/10.1134/S0036023613120140
I. N. Klyukin, A. S. Kubasov, I. P. Limarev, et al., Polyhedron 101, 215 (2015). https://doi.org/10.1016/j.poly.2015.09.025
I. N. Klyukin, A. P. Zhdanov, E. Y. Matveev, et al., Inorg. Chem. Commun. 50, 28 (2014). https://doi.org/10.1016/j.inoche.2014.10.008
E. Y. Matveev, A. S. Kubasov, G. A. Razgonyaeva, et al., Russ. J. Inorg. Chem. 60, 776 (2015). https://doi.org/10.1134/S0036023615070104
A. S. Kubasov, E. Y. Matveev, E. S. Turyshev, et al., Dokl. Chem. 477, 257 (2017). https://doi.org/10.1134/S0012500817110088
A. P. Zhdanov, K. A. Zhdanova, A. Y. Bykov, et al., Polyhedron 139, 125 (2018). https://doi.org/10.1016/j.poly.2017.09.050
I. N. Klyukin, A. P. Zhdanov, A. Yu. Bykov, et al., Russ. J. Inorg. Chem. 63, 213 (2018). https://doi.org/10.1134/S0036023618020110
A. P. Zhdanov, M. V. Lisovsky, L. V. Goeva, et al., Russ. Chem. Bull. 58, 1694 (2009). https://doi.org/10.1007/s11172-009-0234-9
A. L. Mindich, N. A. Bokach, M. L. Kuznetsov, et al., ChemplusChem. 77, 1075 (2012). https://doi.org/10.1002/cplu.201200257
K. A. Zhdanova, A. P. Zhdanov, A. V. Ezhov, et al., Russ. Chem. Bull. 63, 194 (2014). https://doi.org/10.1007/s11172-014-0413-1
K. A. Zhdanova, A. P. Zhdanov, A. V. Ezhov, et al., Macroheterocycles 7, 394 (2014). https://doi.org/10.6060/mhc140494z
M. Y. Losytskyy, V. B. Kovalska, O. A. Varzatskii, et al., J. Lumin. 169, 51 (2016). https://doi.org/10.1016/j.jlumin.2015.08.042
D. S. Bolotin, V. K. Burianova, A. S. Novikov, et al., Organometallics 35, 3612 (2016). https://doi.org/10.1021/acs.organomet.6b00678
A. P. Zhdanov, I. N. Klyukin, A. Y. Bykov, et al., Polyhedron 123, 176 (2017). https://doi.org/10.1016/j.poly.2016.11.035
D. S. Bolotin, M. Y. Demakova, E. A. Daines, et al., Russ. J. Gen. Chem. 87, 37 (2017). https://doi.org/10.1134/S107036321701008X
A. P. Zhdanov, A. Y. Bykov, A. S. Kubasov, et al., Russ. J. Inorg. Chem. 62, 468 (2017). https://doi.org/10.1134/S0036023617040210
V. K. Burianova, A. S. Mikherdov, D. S. Bolotin, et al., J. Organomet. Chem. 870, 97 (2018). https://doi.org/10.1016/j.jorganchem.2018.06.017
E. A. Daines, D. S. Bolotin, N. A. Bokach, et al., Inorg. Chim. Acta 471, 372 (2018). https://doi.org/10.1016/j.ica.2017.11.054
V. K. Burianova, D. S. Bolotin, A. S. Mikherdov, et al., New J. Chem. 42, 8693 (2018). https://doi.org/10.1039/c8nj01018h
A. L. Mindich, N. A. Bokach, F. M. Dolgushin, et al., Organometallics 31, 1716 (2012). https://doi.org/10.1021/om200993f
A. L. Mindich, N. A. Bokach, M. L. Kuznetsov, et al., Organometallics 32, 6576 (2013). https://doi.org/10.1021/om400892x
SAINT, Version 7.23A (Bruker, 2003).
SADABS-2004/1 (Bruker, 2004).
G. M. Sheldrick, Acta Crystallogr., Sect. A 64, 112 (2008). https://doi.org/10.1107/S0108767307043930
G. M. Sheldrick, Acta Crystallogr. Sect. A. Found. Crystallogr. 71, 3 (2015). https://doi.org/10.1107/S2053273314026370
I. B. Sivaev, N. A. Votinova, V. I. Bragin, et al., J. Organomet. Chem. 657, 163 (2002). https://doi.org/10.1016/S0022-328X0201419-5
A. P. Zhdanov, I. N. Polyakova, G. A. Razgonyaeva, et al., Russ. J. Inorg. Chem. 56, 847 (2011). https://doi.org/10.1134/S003602361106026X
M. Y. Losytskyy, V. B. Kovalska, O. A. Varzatskii, et al., J. Lumin. 169, 51 (2016). https://doi.org/10.1016/j.jlumin.2015.08.042
A. V. Ezhov, F. Y. Vyal’ba, I. N. Kluykin, et al., Macroheterocycles 10, 505 (2017). https://doi.org/10.6060/mhc171254z
J. F. W. McOmie, Protective Groups in Organic Chemistry (Springer, Boston, MA, 1995). https://doi.org/10.1007/978-1-4684-7218-9
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, et al., J. Appl. Crystallogr. 42, 339 (2009). https://doi.org/10.1107/S0021889808042726
F. H. Allen, O. Kennard, D. G. Watson, et al., J. Chem. Soc., Perkin Trans. 2, 1 (1987) https://doi.org/10.1039/p298700000s1
S. Z. Konieczka, A. Himmelspach, M. Hailmann, et al., Eur. J. Inorg. Chem., No. 1, 134 (2013). https://doi.org/10.1002/ejic.201200969
F. Li, K. Shelly, C. B. Knobler, et al., Inorg. Chem. 38, 4926 (1999). https://doi.org/10.1021/ic990744h
D. B. Bryan, R. F. Hall, K. G. Holden, et al., J. Am. Chem. Soc. 99, 2353 (1977). https://doi.org/10.1021/ja00449a063
F. Alam, A. H. Soloway, R. F. Barth, et al., J. Med. Chem. 32, 2326 (1989). https://doi.org/10.1021/jm00130a017
ACKNOWLEDGMENTS
The studies were conducted using the facilities of the Shared Physical Analysis Facilities Center of the Kurnakov Institute of General and Inorganic Chemistry, which functions under the State Assignment to the Kurnakov Institute in the field of basic research.
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 19-03-00218_a) and the Council of Russian Federation Presidential Grants (project nos. MK-2403.2019.3 and NSh-2845.2018.3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article summarizes the results of the research paper competition held under the IX Conference of Young Scientists on General and Inorganic Chemistry (Kurnakov Institute of General and Inorganic Chemistry, Moscow, Russia, 2019).
Additional information
Translated by D. Terpilovskaya
Rights and permissions
About this article
Cite this article
Nelyubin, A.V., Klyukin, I.N., Zhdanov, A.P. et al. Synthesis of Substituted Derivatives of closo-Decaborate Anion with a Peptide Bond: The Way towards Designing Biologically Active Boron-Containing Compounds. Russ. J. Inorg. Chem. 64, 1499–1506 (2019). https://doi.org/10.1134/S003602361912012X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602361912012X