Abstract
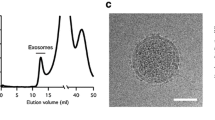
Exosomes are cell-derived vesicles that are secreted by both normal and cancer cells. Over the last decade, a few studies have revealed that exosomes cross talk and/or influence major tumor-related pathways such as angiogenesis and metastasis involving many cell types within the tumor microenvironment. The protein composition of the membrane of an exosome reflects that of the membrane of the cell of origin. Because of this, tumor-derived exosomes differ from exosomes that are derived from normal cells. The detection of tumor exosomes and analysis of their molecular composition hold promise for diagnosis and prognosis of cancer. Here, we present hydrogel microarrays (biochips), which contain a panel of immobilized antibodies that recognize tetraspanins (CD9, CD63, CD81) and prognostic markers for colorectal cancer (A33, CD147). These biochips make it possible to analyze the surface proteins of either isolated exosomes or exosomes that are present in the serum samples without isolation. These biochips were successfully used to analyze the surface proteins of exosomes from serum that was collected from a colorectal cancer patient and healthy donor. Biochip-guided immunofluorescent analysis of the exosomes has made it possible for us to detect the A33 antigen and CD147 in the serum sample of the colorectal cancer patient with normal levels of CEA and CA19-9.
Similar content being viewed by others
References
Iraci N., Leonardi T., Gessler F., et al. 2016. Focus on extracellular vesicles: Physiological role and signalling properties of extracellular membrane vesicles. Int. J. Mol. Sci. 17, 171.
Li J., Liu K., Liu Y., et al. 2013. Exosomes mediate the cell-to-cell transmission of IFN-induced antiviral activity. Nat. Immunol. 14, 793–803.
Cossetti C., Iraci N., Mercer T.R., et al. 2014. Extracellular vesicles from neural stem cells transfer IFN-via Ifngr1 to activate Stat1 signaling in target cells. Mol. Cell. 56, 193–204.
Bobrie A., Colombo M., Raposo G., et al. 2011. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic. 12, 1659–1668.
Mittelbrunn M., Gutierrez-Vazquez C., Villarroya-Beltri C., et al. 2011. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigenpresenting cells. Nat. Commun. 2, 282.
Johnstone R.M., Adam M., Hammond J.R., et al. 1987. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 262, 9412–9420.
Raposo G., Stoorvogel W. 2013. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383.
Zoller M. 2009. Tetraspanins: Push and pull in suppressing and promoting metastasis. Nat. Rev. Cancer. 9, 40–55.
Rana S., Zoller M. 2011. Exosome target cell selection and the importance of exosomal tetraspanins: A hypothesis. Biochem. Soc. Trans. 39, 559–562.
Martínez Z.A., Yáñez-Mó M. 2014. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 5, 442.
Keerthikumar S., Gangoda L., Gho Y.S., Mathivanan S. 2017. Bioinformatics tools for extracellular vesicles research. Meth. Mol Biol. 1545, 189–196.
Al-Nedawi K., Meehan B., Rak J. 2009. Microvesicles: Messengers and mediators of tumor progression. Cell Cycle. 8, 2014–2018.
Martins V.R., Dias M.S., Hainaut P. 2013. Tumor-cellderived microvesicles as carriers of molecular information in cancer. Curr. Opin. Oncol. 1, 66–75.
Hoshino A., Costa-Silva B., Shen T., et al. 2015. Tumour exosome integrins determine organotropic metastasis. Nature. 527 (7578), 329–335.
Rak J. 2015. Cancer: Organ-seeking vesicles. Nature. 527 (7578), 312314.
Simpson R.J., Lim J.W., Moritz R.L., et al. 2009. Exosomes: Proteomic insights and diagnostic potential. Expert. Rev. Proteomics. 6, 267–283.
Jia S., Zocco D., Samuels M.L., et al. 2014. Emerging technologies in extracellular vesicle-based molecular diagnostics. Expert Rev. Mol. Diagn. 14 (3), 307–321.
Street J.M., Yuen P.S., Star R.A. 2014. Bioactive exosomes: Possibilities for diagnosis and management of bladder cancer. J. Urol. 192 (2), 297–306.
Sheridan C. 2016. Exosome cancer diagnostic reaches market. Nat. Biotechnol. 34, 359–360.
Witwer K.W., Buzás E.I., Bemis L.T., et al. 2013. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles. 2, 20360–20385.
Thery C., Amigorena S., Raposo G., et al. 2006. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. Ch. 3, Unit 3.22.
Rubina A.Y., Dementieva E.I., Stomakhin A.A., et al. 2003. Hydrogel-based protein microchips: Manufacturing, properties, and applications. Biotechniques. 34, 1008–1022.
Hermanson G.T. 1996. Bioconjugate Techniques. San Diego, CA: Academic Press.
Luga V., Zhang L., Viloria-Petit A.M., et al. 2012. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 151, 1542–1556.
Smyth T.J., Redzic J.S., Graner M.W., et al. 2014. Examination of the specificity of tumor cell derived exosomes with tumor cells in vitro. Biochim. Biophys. Acta. 1838, 2954–2965.
Yue S., Mu W., Erb U., Zöller M. 2015. The tetraspanins CD151 and Tspan8 are essential exosome components for the crosstalk between cancer initiating cells and their surrounding. Oncotarget. 6, 2366–2384.
Zarovni N., Corrado A., Guazzi P., et al. 2015. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods. 87, 46–58.
Taylor D.D., Zacharias W., Gercel-Taylor C. 2011. Exosome isolation for proteomic analyses and RNA profiling. Meth. Mol. Biol. 728, 235–246.
Belov L., Matic K.J., Hallal S., et al. 2016. Extensive surface protein profiles of extracellular vesicles from cancer cells may provide diagnostic signatures from blood samples. J. Extracell. Vesicles. 5, 1–12.
Vlassov A.V., Magdaleno S., Setterquist R., et al. 2012. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta. 1820, 940–948.
Sakamoto J., Kojima H., Kato J., et al. 2000. Organspecific expression of the intestinal epithelium-related antigen A33, a cell surface target for antibody-based imaging and treatment in gastrointestinal cancer. Cancer Chemother. Pharmacol. 46, Suppl: S27–32.
Ritter G., Cohen L.S., Williams C. Jr., et al. 2001. Serological analysis of human anti-human antibody responses in colon cancer patients treated with repeated doses of humanized monoclonal antibody A33. Cancer Res. 61 (18), 6851–6859.
Tian X., Ye C., Yang Y., et al. 2015. Expression of CD147 and matrix metalloproteinase-11 in colorectal cancer and their relationship to clinicopathological features. J. Transl. Med. 13, 337.
Welt S., Divgi C.R., Real F.X., et al. 1990. Quantitative analysis of antibody localization in human metastatic colon cancer: A Phase I study of monoclonal antibody A33. J. Clin. Oncol. 8, 1894–1906.
Garin-Chesa P., Sakamoto J., Welt S., et al. 1996. Organ-specific expression of the colon cancer antigen A33, a cell surface target for antibody-based therapy. Int. J. Oncol. 9, 465–471.
Daghighian F., Barendswaard E., Welt S., et al. 1996. Enhancement of radiation dose to the nucleus by vesicular internalization of iodine-125-labeled A33 monoclonal antibody. J. Nucl. Med. 37, 1052–1057.
Mathivanan S., Lim J.W., Tauro B.J., et al. 2010. Proteomics analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol. Cell. Proteomics. 9 (2), 197–208.
Yoshioka Y., Kosaka N., Konishi Y., et al. 2014. Ultrasensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat. Commun. 5, 3591.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.I. Butvilovskaya, A.A. Tikhonov, E.N. Savvateeva, A.A. Ragimov, E.L. Salimov, S.A. Voloshin, D.V. Sidorov, M.A. Chernichenko, A.P. Polyakov, M.M. Filushin, M.V. Tsybulskaya, A.Yu. Rubina, 2017, published in Molekulyarnaya Biologiya, 2017, Vol. 51, No. 5, pp. 817–823.
Rights and permissions
About this article
Cite this article
Butvilovskaya, V.I., Tikhonov, A.A., Savvateeva, E.N. et al. Hydrogel microchip as a tool for studying exosomes in human serum. Mol Biol 51, 712–717 (2017). https://doi.org/10.1134/S0026893317050053
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893317050053