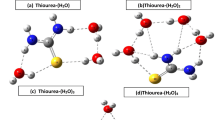
Abstract
The equimolar complexation behavior of urea or thiourea with 2-alcoxybenzamides has been studied by theoretical calculations. Structural models for the calculation were constructed from the X-ray crystallographic structures of 2-methoxybenzamide (MB) crystal and 2-ethoxybenzamide (EB)-thiourea, MB-thiourea, and MB-urea equimolar cocrystals. Structural optimization for EB—urea equimolar cocrystal was performed by the density functional theory (DFT) method (B3LYP/6-31G** level) and the complexation energy was determined using the DFT with higher order basis set (6–31+G**). Energetic stabilization by the equimolar complexation was observed in the three equimolar complexes. The reason why the amide group of MB is out-of-plane in unprocessed MB crystals is well explained by the calculations. It was suggested that intermolecular hydrogen bonding increases in the out-of-plane structure of MB and that subsequently leads to stabilization in the crystal. The amide group of MB or EB was inplane by the complex formation with urea or thiourea. Finally, we predict the possibility of EB-urea equimolar complex formation in terms of the complexation energy.
Similar content being viewed by others
References
K. Takemoto and N. Sonoda, in: Inclusion Compounds Vol. 2, J. L. Atwood, J. E. D. Davies and D.D. MacNicol (eds), Academic Press, L. (1984).
P. Jara, J. Merchan, N. Yutronic, and G. Gonzalez, J. Incl. Phenom. Macro., 36, 101/102 (2000).
A. R. George and K. D. M. Harris, J. Mol. Graph., 13, 138–141 (1995).
K. D. M. Harris, J. Mol. Struct., 374, 241–250 (1996).
M. Brandstätter and A. Burger, J. Therm. Anal., 50, 559–567 (1997).
C. C. Rusa, C. Luca, A. E. Tonelli, and M. Rusa, Polymer, 43, 3969–3972 (2002).
K. D. M. Harris and J. M. Thomas, J. Chem. Soc. Faraday Trans., 86, 2985–2996 (1990).
G. H. Penner, J. M. Polson, C. Stuart, G. Ferguson, and B. J. Kaitner, J. Phys. Chem., 96, 5121–5129 (1992).
T. C. W. Mak, O. W. Law, M. F. C. Ladd, and D. C. Povey, Acta Crystall. B, 34, 1290–1294 (1978).
N. Yutronic, V. Manriquez, P. Jara, O. Witke, J. Merchan, and G. González, J. Chem. Soc. Perkin Trans., 2, 1757–1760 (2000).
M. D. Hollingsworth, B. D. Santarsiero, H. Oumar-Mahamat, and C. J. Nichols, Chem. Mater., 3, 23–35 (1991).
V. Videnova-Adrabińska, J. Mol. Struct., 374, 199–222 (1996).
K. Moribe, M. Tsuchiya, Y. Tozuka, K. Yamaguchi, T. Oguchi, and K. Yamamoto, Chem. Pharm. Bull., 52, 524–539 (2004).
K. Moribe, M. Tsuchiya, Y. Tozuka, K. Yamaguchi, T. Oguchi, and K. Yamamoto, J. Incl. Phenom. Macro., 54, 9–16 (2006).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, Jr. J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian 09, Revision B.01., Gaussian, Inc., Wallingford CT, USA (2010).
A. D. Becke, J. Chem. Phys., 98, 5648–5652 (1993).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B, 37, 785–789 (1988).
S. Simon, M. Duran, and J. J. Dannenberg, J. Chem. Phys., 105, 11024–11031 (1996).
S. F. Boys and F. Bernardi, Mol. Phys., 19, 553–566 (1970).
M. Hata, M. Tsuda, N. Fujii, and S. Oikawa, Appl. Surf. Sci., 79/80, 255–263 (1994).
M. Hata, Y. Murayama, T. Hoshino, and M. Tsuda, Appl. Surf. Sci., 130–132, 689–693 (1998).
M. V. Vener, A. N. Egorova, A. V. Churakov, and V. G. Tsirelson, J. Comput. Chem., 33, 2303–2309 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2014 M. Hata, K. Moribe, S. Ando, Y. Tozuka, K. Yamamoto.
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 55, Supplement 2, pp. S329–S336, 2014.
Rights and permissions
About this article
Cite this article
Hata, M., Moribe, K., Ando, S. et al. Density functional theory study of equimolar complexation of urea or thiourea with 2-alcoxybenzamide. J Struct Chem 55, 1506–1513 (2014). https://doi.org/10.1134/S0022476614080186
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476614080186