Abstract
Background
Several articles showed that statistical efficiency of multi-arm randomized clinical trials (RCTs) is much better than conventional two-arm RCTs. Multi-arm RCTs attract interest mainly when the experimental treatment regimen is not optimized or several pipelines under development exist. Breast cancer is a possible candidate disease. Our aim was to elucidate the current study designs and multiplicity adjustment methods in multi-arm RCTs.
Methods
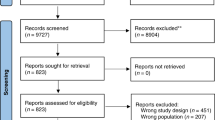
A search of the PubMed database revealed 468 articles on breast cancer RCTs published from 2010 to 2016. Information on study designs and analysis methods was collected from 4 major journals.
Results
A total of 202 RCTs were selected, 48 were multi-arm and 29 were three-arm RCTs. In two of the target journals, multi-arm RCTs have been increasingly reported since 2013. Compared with two-arm RCTs, three-arm RCTs were frequently conducted in neoadjuvant settings (7.7% vs 33.3%). The number of trials performed in perioperative settings was 46 in two-arm and 15 in three-arm RCTs. Of these, the proportion of industry-sponsored trials in two-arm and three-arm RCTs was 26.1% and 53.3%, respectively. Shared control designs (SCDs) which randomized to a common control arm and multiple experimental arms comprised 54.2% of 48 multi-arm RCTs. For SCDs, detailed information on multiplicity adjustment methods was seldom reported. The Bonferroni adjustment method together with alpha-spending functions was commonly used.
Conclusion
Breast cancer multi-arm RCTs have been increasingly reported. The majority of multi-arm RCTs are industry-sponsored trials using SCDs in neoadjuvant settings. Detailed description about multiplicity adjustment methods is required for multi-arm RCTs.


Similar content being viewed by others
References
Parmar MK, Carpenter J, Sydes MR. More multiarm randomised trials of superiority are needed. The Lancet. 2014;384:283–4.
Freidlin B, Korn EL, Gray R, et al. Multi-arm clinical trials of new agents: some design considerations. Clin Cancer Res. 2008;14:4368–71.
Dmitrienko A, D'Agostino R Sr. Traditional multiplicity adjustment methods in clinical trials. Stat Med. 2013;32:5172–218.
Dmitrienko A, D'Agostino RB Sr, Huque MF. Key multiplicity issues in clinical drug development. Stat Med. 2013;32:1079–111.
Huque MF, Dmitrienko A, D’Agostino R. Multiplicity issues in clinical trials with multiple objectives. Stat in Biopharm Res. 2013;5:321–37.
Dmitrienko A, D’Agostino RB Sr. Multiplicity considerations in clinical trials. N Engl J Med. 2018;378:2115–222.
Parmar MK, Barthel FMS, Sydes M, et al. Speeding up the evaluation of new agents in cancer. J Natl Cancer Inst. 2008;100:1204–14.
EMA (2002) Points to consider on multiplicity issues in clinical trials. The European Medicine Agency
EMA (2016) Guideline on multiplicity issues in clinical trials (draft version)
US FDA. Multiple endpoints in clinical trials: guidance for industry (draft version). Silver Spring: U.S. Food and Drug Administration; 2017.
Sherman RE, Anderson SA, Dal Pan GJ, et al. Real-world evidence—what is it and what can it tell us. N Engl J Med. 2016;375:2293–7.
Sherman RE, Davies KM, Robb MA, et al. Accelerating development of scientific evidence for medical products within the existing US regulatory framework. Nat Rev Drug Discov. 2017;16:297.
Woodcock J, LaVange LM. Master protocols to study multiple therapies, multiple diseases, or both. N Engl J Med. 2017;377:62–70.
US FDA (2017) Work plan and proposed funding allocations of FDA innovation account.
Baron G, Perrodeau E, Boutron I, et al. Reporting of analyses from randomized controlled trials with multiple arms: a systematic review. BMC Med. 2013;11:84.
Wason JM, Stecher L, Mander AP. Correcting for multiple-testing in multi-arm trials: is it necessary and is it done? Trials. 2014;15:364.
WHO primary registries of international clinical trials registry platform (ICTRP). World Health Organization
US FDA. Pathological complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an end point to support accelerated approval. Silver Spring: U.S Food and Drug Administration; 2014.
Simon R, Wittes R, Ellenberg S. Randomized phase II clinical trials. Cancer Treat Rep. 1985;69:1375–81.
Stockler MR, Harvey VJ, Francis PA, et al. Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol. 2011;29:4498–504.
Perez EA, Suman VJ, Davidson NE, et al. Sequential versus concurrent trastuzumab in adjuvant chemotherapy for breast cancer. J Clin Oncol. 2011;29:4491.
Wason JM, Jaki T, Stallard N. Planning multi-arm screening studies within the context of a drug development program. Stat Med. 2013;32:3424–35.
Juszczak E, Altman DG, Hopewell S, et al. Reporting of multi-arm parallel-group randomized trials: extension of the CONSORT 2010 statement. JAMA. 2019;321:1610–20.
Moher D, Schulz KF, Altman D. The consort statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987–91.
Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134:663–94.
Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869.
Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332.
Kantarjian HM, Fojo T, Mathisen M, et al. Cancer drugs in the United States: Justum Pretium—the just price. J Clin Oncol. 2013;31:3600–4.
Kocher R, Roberts B. The calculus of cures. N Engl J Med. 2014;370:1473–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SN reports personal fees from Taiho, AstraZeneca, Chugai, and Pfizer, outside the submitted work. YA is the employee of Shionogi & Co., Ltd.
Additional information
Presented at the 2018 Eastern North American Region Spring Meeting, March 25–28, 2018, Atlanta, United States.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nomura, S., Miyauchi, Y., Ajisawa, Y. et al. Study Designs in Multi-arm Trials for Breast Cancer: A Systematic Literature Review of Major Journals. Ther Innov Regul Sci 54, 1185–1191 (2020). https://doi.org/10.1007/s43441-020-00141-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-020-00141-3