Abstract
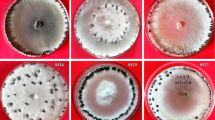
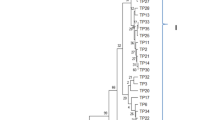
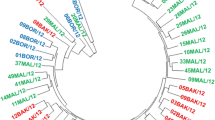
Sclerotinia sclerotiorum is a newly emerging phytopathogen in Bangladesh causing white mold disease in many plants, including important horticultural and field crops. In this study, we isolated S. sclerotiorum strains from infected parts of various host crops and characterized them using morphophysiological and genetic approaches. White mold of mustard; pod rot or fruit rot of bush bean and garden pea; head rot of cauliflower; flower rot or blossom rot of rose and Salvia; fruit rot of squash; inflorescence rot and pod rot of country bean; and leaf drop or rot of coriander and lettuce were observed in several regions of Bangladesh. From the infected crops, a total of 36 fungal strains were isolated and identified as S. sclerotiorum using internal transcribed spacer (ITS) sequencing. The S. sclerotiorum isolates showed white to off-white mycelial growth with loose to dense velvety aerial mycelia. Round to irregular shaped sclerotia formation was observed, with 4-38 sclerotia present per Petri plate. Apothecia formation from sclerotia was also noted under both natural and artificial conditions. In the present study, as many as 13 crops: Cosmos bipinnatus, Amaranthus cruentus, Leucas aspera, Glycine max, Dahlia hortensis, Hibiscus rosa-sinensis, Rosa chinensis, Salvia officinalis, Cucurbita pepo, Lagenaria siceraria, Brassica oleracea var. italica, Coriandrum sativum, and Lactuca sativa were identified as new host crops of S. sclerotiorum in Bangladesh based on morphological characteristics and ITS sequences.








Similar content being viewed by others
References
Abawi GS, Grogan RG (1979) Epidemiology of Diseases caused by Sclerotinia Species. Phytopathology 69:899–904
Abreu MJ, Souza EA (2015) Investigation of Sclerotinia sclerotiorum strains variability in Brazil. Genet Mol Res 14(2):6879–6896
Adams PB, Ayers WA (1979) Ecology of Sclerotinia species. Phytopathology 69:896–899
Ahmed AU, Akhond MAY (2015) First report of Sclerotinia rot caused by Sclerotinia sclerotiorum on Lens culinaris in Bangladesh. New Dis Rep 31:23. https://doi.org/10.5197/j.2044-0588.2015.031.023
Aldrich-Wolfe L, Travers S, Nelson BD Jr (2015) Genetic Variation of Sclerotinia sclerotiorum from Multiple Crops in the North Central United States. PLoS One 10(9):e0139188. https://doi.org/10.1371/journal.pone.0139188
Attanayake RN, Porter L, Johnson DA, Chen W (2012) Genetic and phenotypic diversity and random association of DNA markers of isolates of the fungal plant pathogen Sclerotinia sclerotiorum from soil on a fine geographic scale. Soil Biol Biochem 55:28–36
Blad B, Steadman JR, Weiss A (1978) Canopy structure and irrigation influence white mold disease and microclimate of dry edible beans. Phytopathology 68:1431–1437
Boland GJ, Hall R (1994) Index of plant hosts of Sclerotinia sclerotiorum. Can J Plant Pathol 16:93–10
Bolton MD, Thomma BPHJ, Nelson BD (2006) Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen. Mol Plant Pathol 7:1–16
Carbone I, Kohn LM (1993) Ribosomal DNA sequence divergence within internal transcribed spacer 1 of the Sclerotiniaceae. Mycologia 85:415–427
Dey TK, Hossain MS, Walliwallah H, Islam K, Islam S, Hoque E (2008) Sclerotinia sclerotiorum: An emerging threat for crop production. BSPC, BARI, Bangladesh, pp 12
Freeman J, Ward E, Calderon C, McCartney A (2002) A polymerase chain reaction (PCR) assay for the detection of inoculum of Sclerotinia sclerotiorum. Eur J Plant Pathol 108:877–886
Hambleton S, Walker C, Kohn LM (2002) Clonal lineages of Sclerotinia sclerotiorum previously known from other crops predominate in 1999–2000 samples from Ontario and Quebec soybean. Can J Plant Pathol 24:309–315
Hossain MD, Rahman MME, Islam MM, Rahman MZ (2008) White rot, a new disease of mustard in Bangladesh. Bangladesh J Plant Pathol 24:81–82
Kohli Y, Morrall RAA, Anderson JB, Kohn LM (1992) Local and trans Canadian clonal distribution of Sclerotinia sclerotiorum on canola. Phytopathology 82:875–880
Kohn LM, Stasovski E, Carbone I, Royer J, Anderson JB (1991) Mycelial incompatibility and molecular markers identify genetic variability in field populations of Sclerotinia sclerotiorum. Phytopathology 81:480–485
Kull LS, Pedersen WL, Palmquist D, Hartman GL (2004) Mycelial compatibility grouping and aggressiveness of Sclerotinia sclerotiorum. Plant Dis 88:325–332
Li Z, Wang Y, Chen Y, Zhang J, Fernando WGD (2009) Genetic diversity and differentiation of Sclerotinia sclerotiorum populations in sunflower. Phytoparasitica 37:77–85
Mandal AK, Dubey SC (2012) Genetic diversity analysis of Sclerotinia sclerotiorum causing stem rot in chickpea using RAPD, ITS-RFLP, ITS sequencing and mycelial compatibility grouping. World J Microbiol Biotechnol 28:1849–1855.
Njambere EN, Peever TL, Vandemark G, Chen W (2014) Genotypic variation and population structure of Sclerotinia trifoliorum infecting chickpea in California. Plant Pathol 63:994–100
Prova A, Akanda MAM, Islam S, Sultana F, Islam MT, Hossain MM (2014) First report of stem and pod blight of hyacinth bean caused by Sclerotinia sclerotiorum in Bangladesh. J Plant Pathol 96(3):607
Purdy LH (1979) Sclerotinia sclerotiorum: History, diseases and symptomatology, host range, geographic distribution and impact. Phytopathology 69:875–880
Rahman MME, Dey TK, Hossain DM, Nonaka M, Harada N (2015a) First report of white mold caused by Sclerotinia sclerotiorum on jackfruit. Australas Plant Dis Notes 10:10. https://doi.org/10.1007/s13314-014-0155-9
Rahman MME, Hossain DM, Dey TK, Sarker SR, Nonaka M, Harada N (2015b) First report of white mould caused by Sclerotinia sclerotiorum on marigold (Tagetes erecta) in Bangladesh. J Plant Pathol 97(2):398
Schwartz HF, Steadman JR (1978) Factors affecting sclerotium populations of, and apothecium production by, Sclerotinia sclerotiorum. Phytopathology 68:383–388
Sexton AC, Howlett BJ (2004) Microsatellite markers reveal genetic differentiation among populations of Sclerotinia sclerotiorum from Australian canola fields. Curr Genet 46:357–365
Shaner G, Finney RE (1977) The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox wheat. Phytopathology 67:1051–1056
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols. A Guide to Methods and Applications. Academic Press, San Diego, USA, pp 315–322
Yli-Mattila T, Kalko G, Hannukkala A, Paavanen-Huhtala S, Hakala K (2010) Prevalence, species composition, genetic variation and pathogenicity of clover rot (Sclerotinia trifoliorum) and Fusarium spp. in red clover in Finland. Eur J Plant Pathol 126:13–27
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rahman, M.M.E., Suzuki, K., Islam, M.M. et al. Molecular characterization, mycelial compatibility grouping, and aggressiveness of a newly emerging phytopathogen, Sclerotinia sclerotiorum, causing white mold disease in new host crops in Bangladesh. J Plant Pathol 102, 775–785 (2020). https://doi.org/10.1007/s42161-020-00503-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-020-00503-8