Abstract
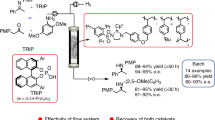
Asymmetric homogeneous hydrogenation under high pressure in continuous flow was achieved with a slug flow reactor. High hydrogen pressure enabled iridium-catalyzed asymmetric hydrogenation of acetophenone with a turn-over-frequency (TOF) of up to 274,000 h−1. An operando infrared tool was used to provide in-situ monitoring of the reaction. The effect of gas-liquid ratio and speed of slug flow in the microchannel were studied. The multi-step flow synthesis of active pharmaceutical ingredients, in which asymmetric hydrogenation is a key step, was successfully demonstrated, with subsequent reactions carried out under longer residence times within a cascade of CSTRs.







Similar content being viewed by others
References
Adamo A, Beingessner RL, Behnam M, Chen J, Jamison TF, Jensen KF, Monbaliu J-CM, Myerson AS, Revalor EM, Snead DR, Stelzer T, Weeranoppanant N, Wong SY, Zhang P (2016) On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science 352(6281):61
Cole KP, Groh JM, Johnson MD, Burcham CL, Campbell BM, Diseroad WD, Heller MR, Howell JR, Kallman NJ, Koenig TM, May SA, Miller RD, Mitchell D, Myers DP, Myers SS, Phillips JL, Polster CS, White TD, Cashman J, Hurley D, Moylan R, Sheehan P, Spencer RD, Desmond K, Desmond P, Gowran O (2017) Kilogram-scale prexasertib monolactate monohydrate synthesis under continuous-flow CGMP conditions. Science 356(6343):1144
Russell MG, Jamison TF (2019) Seven-Step Continuous Flow Synthesis of Linezolid Without Intermediate Purification. Angew Chem Int Ed 58(23):7678–7681
Steiner S, Wolf J, Glatzel S, Andreou A, Granda JM, Keenan G, Hinkley T, Aragon-Camarasa G, Kitson PJ, Angelone D, Cronin L (2019) Organic synthesis in a modular robotic system driven by a chemical programming language. Science 363 (6423):eaav2211
Fülöp Z, Szemesi P, Bana P, Éles J, Greiner I (2020) Evolution of flow-oriented design strategies in the continuous preparation of pharmaceuticals. React Chem Eng 5(9):1527–1555
Chatterjee S, Guidi M, Seeberger PH, Gilmore K (2020) Automated radial synthesis of organic molecules. Nature 579(7799):379–384
Rogers L, Briggs N, Achermann R, Adamo A, Azad M, Brancazio D, Capellades G, Hammersmith G, Hart T, Imbrogno J, Kelly LP, Liang G, Neurohr C, Rapp K, Russell MG, Salz C, Thomas DA, Weimann L, Jamison TF, Myerson AS, Jensen KF (2020) Continuous Production of Five Active Pharmaceutical Ingredients in Flexible Plug-and-Play Modules: A Demonstration Campaign. Org Process Res Dev 24(10):2183–2196
Tsubogo T, Oyamada H, Kobayashi S (2015) Multistep continuous-flow synthesis of (R)- and (S)-rolipram using heterogeneous catalysts. Nature 520(7547):329–332
Rossi S, Porta R, Brenna D, Puglisi A, Benaglia M (2017) Stereoselective Catalytic Synthesis of Active Pharmaceutical Ingredients in Homemade 3D-Printed Mesoreactors. Angew Chem Int Ed 56(15):4290–4294
Ötvös SB, Pericàs MA, Kappe CO (2019) Multigram-scale flow synthesis of the chiral key intermediate of (−)-paroxetine enabled by solvent-free heterogeneous organocatalysis. Chem Sci 10(48):11141–11146
Saito Y, Kobayashi S (2020) Development of Robust Heterogeneous Chiral Rhodium Catalysts Utilizing Acid–Base and Electrostatic Interactions for Efficient Continuous-Flow Asymmetric Hydrogenations. J Am Chem Soc 142(39):16546–16551
Ötvös SB, Llanes P, Pericàs MA, Kappe CO (2020) Telescoped Continuous Flow Synthesis of Optically Active γ-Nitrobutyric Acids as Key Intermediates of Baclofen, Phenibut, and Fluorophenibut. Org Lett 22(20):8122–8126
Rylander P (1979) Chapter 1 - Hydrogenation Catalysts, Reactors, and Reaction Conditions. In: Rylander P (ed) The Catalytic Hydrogenation in Organic Syntheses. Academic Press, pp 1–12
Yu T, Jiao J, Song P, Nie W, Yi C, Zhang Q, Li P (2020) Recent Progress in Continuous-Flow Hydrogenation. ChemSusChem 13(11):2876–2893
Yu T, Ding Z, Nie W, Jiao J, Zhang H, Zhang Q, Xue C, Duan X, Yamada YMA, Li P (2020) Recent Advances in Continuous-Flow Enantioselective Catalysis. Chem Eur J 26(26):5729–5747
Künzle N, Mallat T, Baiker A (2003) Enantio selective hydrogenation of isopropyl-4,4,4-trifluoroacetoacetate in a continuous flow reactor. Appl Catalysis A: Gen 238(2):251–257
O’Neal EJ, Lee CH, Brathwaite J, Jensen KF (2015) Continuous Nanofiltration and Recycle of an Asymmetric Ketone Hydrogenation Catalyst. ACS Catal 5(4):2615–2622
Amara Z, Poliakoff M, Duque R, Geier D, Franciò G, Gordon CM, Meadows RE, Woodward R, Leitner W (2016) Enabling the Scale-Up of a Key Asymmetric Hydrogenation Step in the Synthesis of an API Using Continuous Flow Solid-Supported Catalysis. Org Process Res Dev 20(7):1321–1327
Madarász J, Nánási B, Kovács J, Balogh S, Farkas G, Bakos J (2018) Immobilized phosphine–phosphite rhodium complexes: highly active and enantioselective catalysts for asymmetric hydrogenation under continuous flow conditions. Monatsh Chem 149(1):19–25
Yasukawa T, Masuda R, Kobayashi S (2019) Development of heterogeneous catalyst systems for the continuous synthesis of chiral amines via asymmetric hydrogenation. Nat Catal 2(12):1088–1092
Kawakami Y, Borissova A, Chapman MR, Goltz G, Koltsova E, Mitrichev I, Blacker AJ (2019) Continuous Flow Asymmetric Transfer Hydrogenation with Long Catalyst Lifetime and Low Metal Leaching. Eur J Org Chem 2019(45):7499–7505
O'Brien M, Taylor N, Polyzos A, Baxendale IR, Ley SV (2011) Hydrogenation in flow: Homogeneous and heterogeneous catalysis using Teflon AF-2400 to effect gas–liquid contact at elevated pressure. Chem Sci 2(7):1250–1257
Newton S, Ley SV, Arcé EC, Grainger DM (2012) Asymmetric Homogeneous Hydrogenation in Flow using a Tube-in-Tube Reactor. Adv Synth Catal 354(9):1805–1812
Newton S, Carter CF, Pearson CM, de C. Alves L, Lange H, Thansandote P, Ley SV (2014) Accelerating Spirocyclic Polyketide Synthesis using Flow Chemistry. Angew Chem Int Ed 53 (19):4915–4920
Geyer K, Codée JDC, Seeberger PH (2006) Microreactors as Tools for Synthetic Chemists—The Chemists' Round-Bottomed Flask of the 21st Century? Chem Eur J 12(33):8434–8442
Cossar PJ, Hizartzidis L, Simone MI, McCluskey A, Gordon CP (2015) The expanding utility of continuous flow hydrogenation. Org Biomol Chem 13(26):7119–7130
Plutschack MB, Pieber B, Gilmore K, Seeberger PH (2017) The Hitchhiker’s Guide to Flow Chemistry. Chem Rev 117(18):11796–11893
Akwi FM, Watts P (2018) Continuous flow chemistry: where are we now? Recent applications, challenges and limitations. Chem Comm 54(99):13894–13928
Fu WC, Jamison TF (2020) Deuteriodifluoromethylation and gem-Difluoroalkenylation of Aldehydes Using ClCF2H in Continuous Flow. Angew Chem Int Ed 59(33):13885–13890
Abdallah R, Meille V, Shaw J, Wenn D, de Bellefon C (2004) Gas–liquid and gas–liquid–solid catalysis in a mesh microreactor. Chem Comm 4:372–373
de Bellefon C, Lamouille T, Pestre N, Bornette F, Pennemann H, Neumann F, Hessel V (2005) Asymmetric catalytic hydrogenations at micro-litre scale in a helicoidal single channel falling film micro-reactor. Catal Today 110(1):179–187
Guan F, Kapur N, Sim L, Taylor CJ, Wen J, Zhang X, Blacker AJ (2020) A universal reactor platform for batch and flow: application to homogeneous and heterogeneous hydrogenation. React Chem Eng 5(10):1903–1908
Johnson MD, May SA, Calvin JR, Remacle J, Stout JR, Diseroad WD, Zaborenko N, Haeberle BD, Sun W-M, Miller MT, Brennan J (2012) Development and Scale-Up of a Continuous, High-Pressure, Asymmetric Hydrogenation Reaction, Workup, and Isolation. Org Process Res Dev 16(5):1017–1038
Abrams ML, Buser JY, Calvin JR, Johnson MD, Jones BR, Lambertus G, Landis CR, Martinelli JR, May SA, McFarland AD, Stout JR (2016) Continuous Liquid Vapor Reactions Part 2: Asymmetric Hydroformylation with Rhodium-Bisdiazaphos Catalysts in a Vertical Pipes-in-Series Reactor. Org Process Res Dev 20(5):901–910
Johnson MD, May SA, Haeberle B, Lambertus GR, Pulley SR, Stout JR (2016) Design and Comparison of Tubular and Pipes-in-Series Continuous Reactors for Direct Asymmetric Reductive Amination. Org Process Res Dev 20(7):1305–1320
Swagelok fittings and tubes can be found under https://www.swagelok.com/en/product/Fittings
Knauer APG20FG pump
Bronhorst In-Flow F-230MI flow meter
Equilibar Zero flow BPR
Zaiput laboratory scale liquid-liquid separator
Taylor GI (1961) Deposition of a viscous fluid on the wall of a tube. J Fluid Mechanics 10(2):161–165
Triplett KA, Ghiaasiaan SM, Abdel-Khalik SI, Sadowski DL (1999) Gas–liquid two-phase flow in microchannels Part I: two-phase flow patterns. Int J Multiph Flow 25(3):377–394
Shao N, Gavriilidis A, Angeli P (2009) Flow regimes for adiabatic gas–liquid flow in microchannels. Chem Eng Sci 64(11):2749–2761
Mei M, Felis F, Hébrard G, Dietrich N, Loubière K (2020) Hydrodynamics of Gas–Liquid Slug Flows in a Long In-Plane Spiral Shaped Milli-Reactor. Theor Found Chem Eng 54(1):25–47
Radjagobalou R, Blanco J-F, Dechy-Cabaret O, Oelgemöller M, Loubière K (2018) Photooxygenation in an advanced led-driven flow reactor module: Experimental investigations and modelling. Chem Eng Process 130:214–228
Cao Y, Soares C, Padoin N, Noël T (2021) Gas bubbles have controversial effects on Taylor flow electrochemistry. Chem Eng J 406:126811
Bobers J, Grühn J, Höving S, Pyka T, Kockmann N (2020) Two-Phase Flow in a Coiled Flow Inverter: Process Development from Batch to Continuous Flow. Org Process Res Dev 24(10):2094–2104
Kreutzer MT, Kapteijn F, Moulijn JA, Heiszwolf JJ (2005) Multiphase monolith reactors: Chemical reaction engineering of segmented flow in microchannels. Chem Eng Sci 60(22):5895–5916
Günther A, Jensen KF (2006) Multiphase microfluidics: from flow characteristics to chemical and materials synthesis. Lab Chip 6(12):1487–1503
Wu W, Liu S, Duan M, Tan X, Chen C, Xie Y, Lan Y, Dong X-Q, Zhang X (2016) Iridium Catalysts with f-Amphox Ligands: Asymmetric Hydrogenation of Simple Ketones. Org Lett 18(12):2938–2941
Yu J, Duan M, Wu W, Qi X, Xue P, Lan Y, Dong X-Q, Zhang X (2017) Readily Accessible and Highly Efficient Ferrocene-Based Amino-Phosphine-Alcohol (f-Amphol) Ligands for Iridium-Catalyzed Asymmetric Hydrogenation of Simple Ketones. Chem Eur J 23(4):970–975
Kosoglou T, Statkevich P, Johnson-Levonas A, Paolini JF, Bergman AJ, Alton KB (2005) Ezetimibe. Clin Pharmacokinet 44(5):467–494
Sobieszuk P, Aubin J, Pohorecki R (2012) Hydrodynamics and Mass Transfer in Gas-Liquid Flows in Microreactors. Chem Eng Technol 35(8):1346–1358
Heller D, Holz J, Borns S, Spannenberg A, Kempe R, Schmidt U, Börner A (1997) Influence of a remote hydroxy group in the ligand on the reactivity of a chiral hydrogenation catalyst. Tetrahedron: Asymmetry 8(2):213–222
Kraus T, Günther A, de Mas N, Schmidt MA, Jensen KF (2004) An integrated multiphase flow sensor for microchannels. Exp Fluids 36(6):819–832
Chapman MR, Kwan MHT, King G, Jolley KE, Hussain M, Hussain S, Salama IE, González Niño C, Thompson LA, Bayana ME, Clayton AD, Nguyen BN, Turner NJ, Kapur N, Blacker AJ (2017) Simple and Versatile Laboratory Scale CSTR for Multiphasic Continuous-Flow Chemistry and Long Residence Times. Org Process Res Dev 21(9):1294–1301
Yin C, Dong X-Q, Zhang X (2018) Iridium/f-Amphol-catalyzed Efficient Asymmetric Hydrogenation of Benzo-fused Cyclic Ketones. Adv Synth Catal 360(22):4319–4324
Almeida L, Soares-da-Silva P (2007) Eslicarbazepine acetate (BIA 2–093). Neurotherapeutics 4(1):88–96
Ravinder B, Rajeshwar Reddy S, Sridhar M, Murali Mohan M, Srinivas K, Panasa Reddy A, Bandichhor R (2013) An efficient synthesis for eslicarbazepine acetate, oxcarbazepine, and carbamazepine. Tetrahedron Lett 54(22):2841–2844
Acknowledgements
F.G. thanks the joint PhD program of SUStech with the University of Leeds. This project was financially supported by the National Natural Science Foundation of China (21801118) and the Science, Technology and Innovation Commission of Shenzhen (KQTD20150717103157174).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Experimental
High TON AH of acetophenone in flow
To a 5-mL vial was added the catalyst precursor [Ir(COD)Cl]2 (1.4 mg, 0.002 mmol), f-amphox (2.3 mg, 0.0042 mmol) and anhydrous iPrOH (1.0 mL) in an argon-filled glovebox. This vial was sealed and mixed for 2 h at room temperature. tBuOK (89.8 mg, 0.8 mmol) was dissolved in 10 mL anhydrous iPrOH. 0.5 mL catalyst solution was firstly mixed with 10 mL base solution and then acetophenone (9.6 g, 9.4 mL, 80 mmol). The resulting mixture was filtered and the filtrate was added into a flask.
The process diagram was shown in Fig. S7 and Scheme S1. Fig. S8 showed the remote control app and real time IR analysis. The process was washed by anhydrous and degassed iPrOH at a liquid flow rate of 4 mL/min and gas flow rate of 5 sccm (avoid back flow of liquid to gas flow meter) for 5 min and then pressurized the BPR. After the reactor was pressurized to 70 bar and heated to 90 °C, the aforehand reaction medium was pumped instead of solvent. Liquid flow rate was set at 0.23 mL/min and gas flow rate 0.9 mL/min (45 sccm). The reactor system could achieve steady state in 5 reaction volumes. Samples were collected every 15 min in an empty vial. The conversion and ee were analyzed by NMR and HPLC. When reaction finished, system was depressurized by releasing the gas of Equilibar BPR slowly, and washed the whole system by pumping iPrOH for 10 min.
Multi-step flow synthesis of 5
To a 20-mL vial was added the catalyst precursor [Ir(COD)Cl]2 (16.8 mg, 0.025 mmol), f-amphox (31.0 mg, 0.055 mmol) and anhydrous iPrOH (10.0 mL) in an argon-filled glovebox. The solution was mixed for 2 h under room temperature. CsOH•H2O (83.5 mg, 0.5 mmol) was dissolved in 10.0 mL anhydrous iPrOH. 10.0 mL catalyst solution was sequentially mixed with 10.0 mL base solution and 3 (12.5 g, 25 mmol) in 25.0 mL toluene. The resulting mixture was filtrated and clear solution was added into a 50-mL volumetric flask. Then, 5.0 mL iPrOH was added to prepare a 50.0 mL reaction solution.
Deprotection module consisting of 9 fReactors (15.3 mL) were installed after the high-pressure slug flow reactor. The fReactors were filled with 750 mg 10% Pd/C in total. The frit-in-ferrule was installed before the BPR to hold the solid catalyst in the reactor. The hydrogenation module with a residence time of 6.5 min was pressurized to 100 bars and heated to 60 °C. After it reached steady state, the gas liquid flow was directed to the deprotection module without separation. The fReactor module was heated to 80 °C. Two step yield of ezetimibe was 84%. 272 mg product was obtained in 15 min with 95% de.
Multi-step flow synthesis of (R)-9
Medium for module 1
To a 20.0 mL vial was added the catalyst precursor [Ir(COD)Cl]2 (16.8 mg, 0.025 mmol), f-amphol (38.0 mg, 0.055 mmol) and anhydrous iPrOH (10.0 mL) in the argon-filled glovebox. The medium was mixed for 2 h under room temperature. tBuONa (120.0 mg, 1.25 mmol) was dissolved in 5.0 mL catalyst medium. The resulting medium was mixed with 6 (5.2 g, 25 mmol) in anhydrous and degassed 25.0 mL DCM. The resulting medium was filtrated by filter paper and added into a 50 mL volumetric flask. Then, 15.0 mL iPrOH was added to prepare a 50 mL reaction medium.
Medium for module 2
37.5 mL acetic anhydride and 12.5 mL NEt3 were mixed in a 100 mL volumetric flask. 133.0 mg DMAP was dissolved in the medium.
Medium for module 3
Was neat ClSO2NCO.
The module 1 was washed by anhydrous and degassed iPrOH by a liquid flow rate of 4 mL/min and gas flow rate of 5 sccm (avoid back flow of liquid to gas flow meter) for 5 min and then pressurized the BPR. After the pressure and temperature was elevated to 90 bar and 80 °C, the aforehand reaction medium was pumped instead of solvent. Liquid flow rate was 0.46 mL/mins and gas flow rate was 44 sccm. The reaction was monitored by TLC analysis. After it reached steady state with full conversion, the product medium was collected in the gas liquid separator (Schlenk tube in this process). Module 2 consisting of 5 fReactors (8.5 mL) was installed and filled with dry DCM. The medium for module 2 was taken by a 50 mL syringe that was installed on a PhD Ultra syringe pump. Then it was pumped by a flow rate of 0.26 mL/min. The liquid in gas liquid separator was pumped into module 2 by a flow rate of 0.23 mL/min. The hotplate was heated to 70 °C to have a liquid temperature of 60 °C. The residence time of module 2 was 17 mins. The reaction was monitored by TLC analysis. After it reached steady state with full conversion, the outlet was connected to cooling part of the module 3. Neat ClSO2NCO was pumped by a flow rate of 0.069 mL/min and mixed with medium from module 2 in a T-junction. Tubes of module 3 were in a room temperature water bath. The final product was connected in a beaker that has 20 mL water to quench the solution. There was HCl gas generated in module 3. Thus, the calculated residence time was 2.7 min that was longer than batch reaction time that was 1 min. The biphasic medium was left stirring for 1 h. Organic phase was separated. 5 ml DCM was added to wash the aqueous phase for two times. Organic layer was combined and washed with water (5 mL). The solvent was evaporated at 40 °C under reduced pressure. The crude materials were purified by column chromatography. (60 mL 50% ethyl acetate in petroleum ether, then 100 mL 80% ethyl acetate in petroleum ether). In the first run, 320.0 mg eslicarbazepine acetate with 76% yield and 98% ee in 13 min. In the second run, 2.7 g eslicarbazepine acetate with 81% yield and 98% ee in 100 min.
More experimental details could be found in the supplementary information.
Rights and permissions
About this article
Cite this article
Guan, F., Blacker, A.J., Hall, B. et al. High-pressure asymmetric hydrogenation in a customized flow reactor and its application in multi-step flow synthesis of chiral drugs. J Flow Chem 11, 763–772 (2021). https://doi.org/10.1007/s41981-021-00143-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-021-00143-8