Abstract
Background
Physical exercise and nutrition seem to have a key role in the management of hip fracture patients.
Aim
To evaluate the impact of a 2-month rehabilitative protocol combined with dietetic counseling, with or without essential amino acid supplementation, on functioning in hip fracture patients.
Methods
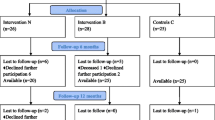
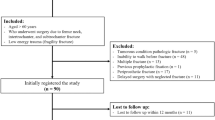
In this pilot randomized controlled study, we recruited patients aged more than 65 years, at 3 months after hip fracture. We randomly assigned the participants into two groups (A and B). Both groups performed a physical exercise rehabilitative programme (five sessions of 40 min/week for 2 weeks, followed by a home-based exercise protocol) and received a dietetic counseling; only group A was supplemented with two sachets of 4 g/day of essential amino acids (Aminotrofic®). We evaluated at baseline and after 2 months of intervention (T1): hand grip strength, Timed Up and Go, and Iowa Level of Assistance scale (ILOA).
Results
The 32 hip fracture patients (mean aged 79.03 ± 7.80 years) were allocated into two groups: group A (n = 16) and group B (n = 16). All the participants showed significant differences in all outcomes at T1 (p < 0.017). Sarcopenic patients in group A (n = 10) showed statistically significant differences in all the primary outcomes at T1 (p < 0.017), whereas sarcopenic patients in group B (n = 13) showed a significant reduction of ILOA only. In non-sarcopenic patients, we found no differences at T1 in all outcome measures.
Discussion
Hip fractures are a complex multifactorial condition of the elderly that determines devastating effects on functioning and independence.
Conclusion
A multidisciplinary rehabilitative and nutritional intervention seems to be effective on functioning in hip fracture patients, in particular sarcopenic ones.



Similar content being viewed by others
References
Tarantino U, Capone A, Planta M et al (2010) The incidence of hip, forearm, humeral, ankle, and vertebral fragility fractures in Italy: results from a 3-year multicenter study. Arthritis Res Ther 12:R226
Roche J, Wenn RT, Sahota O et al (2005) Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: prospective observational cohort study. BMJ 331:1374
Magaziner J, Fredman L, Hawkes W et al (2003) Changes in functional status attributable to hip fracture: a comparison of hip fracture patients to community-dwelling aged. Am J Epidemiol 157:1023–1031
Piscitelli P, Brandi M, Cawston H et al (2014) Epidemiological burden of postmenopausal osteoporosis in Italy from 2010 to 2020: estimations from a disease model. Calcif Tissue Int 95:419–427
Cummings SR, Melton LJ (2002) Epidemiology and outcomes of osteoporotic fractures. Lancet 359:1761–1767
Castelli A, Daidone S, Jacobs R et al (2015) The determinants of costs and length of stay for hip fracture patients. PLoS One 10:e0133545
Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–423
Morley JE (2012) Sarcopenia in the elderly. Fam Pract 29(Suppl 1):i44–i48. https://doi.org/10.1093/fampra/cmr063
Reginster JY, Cooper C, Rizzoli R et al (2016) Recommendations for the conduct of clinical trials for drugs to treat or prevent sarcopenia. Aging Clin Exp Res 28:47–58
Visser M, Harris TB, Fox KM et al (2000) Change in muscle mass and muscle strength after a hip fracture: relationship to mobility recovery. J Gerontol A Biol Sci Med Sci 55:M434–M440
Wehren LE, Hawkes WG, Hebel JR et al (2005) Bone mineral density, soft tissue body composition, strength, and functioning after hip fracture. J Gerontol A Biol Sci Med Sci 60:80–84
Di Monaco M, Castiglioni C, Vallero F et al (2011) Appendicular lean mass does not mediate the significant association between vitamin D status and functional outcome in hip-fracture women. Arch Phys Med Rehabil 92:271–276
Hida T, Ishiguro N, Shimokata H et al (2013) High prevalence of sarcopenia and reduced leg muscle mass in Japanese patients immediately after a hip fracture. Geriatr Gerontol Int 13:413–420
Fiatarone Singh MA (2014) Exercise, nutrition and managing hip fracture in older persons. Curr Opin Clin Nutr Metab Care 17:12–24
Pioli G, Barone A, Giusti A et al (2006) Predictors of mortality after hip fracture: results from 1-year follow-up. Aging Clin Exp Res 18:381–387
Iolascon G, Gimigliano R, Bianco M et al (2017) Are dietary supplements and nutraceuticals effective for musculoskeletal health and cognitive function? A scoping review. J Nutr Health Aging 21:527–538
Roberts KC, Brox WT (2015) AAOS clinical practice guideline: management of hip fractures in the elderly. J Am Acad Orthop Surg 23:138–140
Artaza-Artabe I, Sáez-López P, Sánchez-Hernández N et al (2016) The relationship between nutrition and frailty: effects of protein intake, nutritional supplementation, vitamin D and exercise on muscle metabolism in the elderly. A systematic review. Maturitas 93:89–99
Collins J, Longhurst G, Roschel H et al (2016) Resistance training and co-supplementation with creatine and protein in older subjects with frailty. J Frailty Aging 5:126–134
Rondanelli M, Klersy C, Terracol G et al (2016) Whey protein, amino acids, and vitamin D supplementation with physical activity increases fat-free mass and strength, functionality, and quality of life and decreases inflammation in sarcopenic elderly. Am J Clin Nutr 103:830–840
Finger D, Goltz FR, Umpierre D et al (2015) Effects of protein supplementation in older adults undergoing resistance training: a systematic review and meta-analysis. Sports Med 45:245–255
Pescatello LS (2014) American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription, 9th edn. Wolters Kluwer-Lippincott Williams & Wilkins, Philadelphia
Pfeiffer E (1975) A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc 23:433–441
Linn BS, Linn MW, Gurel L (1968) Cumulative illness rating scale. J Am Geriatr Soc 16:622–626
Janssen I, Heymsfield SB, Baumgartner RN et al (2000) Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol (1985) 89:465–471
Lukaski HC, Johnson PE, Bolanchuk WW et al (1985) Assessment of fat-free mass using bioelectrical impedance measurement of the human body. Am J Clin Nutr 41:810–817
Piccoli A, Rossi B, Pillon L et al (1994) A new method for monitoring body fluid variation by bioimpedance analysis: the RXc graph. Kidney Int 46:534–539
Studenski SA, Peters KW, Alley DE et al (2014) The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 69:547–558
Podsiadlo D, Richardson S (1991) The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39:142–148
Soh SE, Stuart L, Raymond M et al (2017) The validity, reliability, and responsiveness of the modified Iowa Level of Assistance scale in hospitalized older adults in subacute care. Disabil Rehabil 31:1–7
Ware J Jr, Kosinski M, Keller SD (1996) A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 34:220–233
Janssen I, Heymsfield SB, Ross R (2002) Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50:889–896
Ikeda T, Aizawa J, Nagasawa H et al (2016) Effects and feasibility of exercise therapy combined with branched-chain amino acid supplementation on muscle strengthening in frail and pre-frail elderly people requiring long-term care: a crossover trial. Appl Physiol Nutr Metab 41:438–445
WHO (2018) Ageing. http://www.who.int/topics/ageing. Accessed 15 Apr 2018
Kaeberlein M, Rabinovitch PS, Martin GM (2015) Healthy aging: the ultimate preventative medicine. Science 350:1191–1193
Author information
Authors and Affiliations
Contributions
Each author is expected to have made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; or the creation of new software used in the work; or have drafted the work or substantively revised it; and has approved the submitted version (and version substantially edited by journal staff that involves the author’s contribution to the study); and agrees to be personally accountable for the author’s own contributions and for ensuring that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and documented in the literature.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest. Funding sponsors had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, and in the decision to publish the results.
Ethical standards
The experiments comply with the current laws of the country in which they were performed.
Statement of human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Invernizzi, M., de Sire, A., D’Andrea, F. et al. Effects of essential amino acid supplementation and rehabilitation on functioning in hip fracture patients: a pilot randomized controlled trial. Aging Clin Exp Res 31, 1517–1524 (2019). https://doi.org/10.1007/s40520-018-1090-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-018-1090-y