Abstract
Background and aims
Increased adipose tissue may promote catabolic events in skeletal muscle. The aim of this study was to test whether high-fat diet (HFD)-induced obesity would accelerate the onset of muscle wasting in middle-aged mice.
Methods
Muscle was collected from C57BL/6 mice at 9 months of age (baseline) and 14 months of age after consuming a control (C) or HFD. Mice in C and HFD were also subjected to evaluations of body composition and function before and after their respective diets.
Results
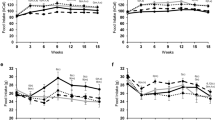
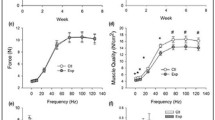
HFD demonstrated significant (p < 0.05) losses of grip strength (−15 %) and sensorimotor coordination (−11 %), whereas C did not. Lean mass decreased to a greater degree in HFD although not significantly (C: −20.69 ± 7.94 vs. HFD: −31.14 ± 5.49 %, p > 0.05). Gastrocnemius, quadriceps, and hamstrings mass in C and HFD were significantly reduced from baseline (−27 to 43 and −39 to 47 %, respectively, p < 0.05) with no differences between the two; however, soleus mass was lower only in HFD (−24 %, p = 0.03). Myofiber area, satellite cells, and myonuclei of the gastrocnemius were lower only in HFD (−23, −19, and −16 %, respectively, p < 0.05) compared to baseline.
Conclusions
HFD-induced obesity adversely affected function in middle-aged mice. Atrophy of the soleus in HFD but not C suggests sensitivity of oxidative muscle to HFD-dependent catabolism more so than aging. In the muscles containing fast/mixed fibers, aging effects may have concealed the catabolic nature of HFD; however, morphological changes in the gastrocnemius including decreased fiber area, satellite cells, and myonuclei are consistent with an atrophic phenotype related to HFD.



Similar content being viewed by others
References
Janssen I, Heymsfield SB, Ross R (2002) Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50(5):889–896
Schrager MA et al (2007) Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol 102(3):919–925
Baumgartner RN (2000) Body composition in healthy aging. Ann N Y Acad Sci 904:437–448
Song MY et al (2004) Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr 79(5):874–880
Cesari M et al (2005) Sarcopenia, obesity, and inflammation–results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr 82(2):428–434
Fontana L et al (2007) Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 56(4):1010–1013
White JP et al (2012) IL-6 regulation on skeletal muscle mitochondrial remodeling during cancer cachexia in the ApcMin/+mouse. Skelet Muscle 2:14
Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, Koniaris LG, Zimmers TA (2012) JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab 303(3):E410–E421
Adechian S et al (2009) Excessive energy intake does not modify fed-state tissue protein synthesis rates in adult rats. Obesity 17(7):1348–1355
Chanseaume E et al (2007) Enhanced muscle mixed and mitochondrial protein synthesis rates after a high-fat or high-sucrose diet. Obesity 15(4):853–859
Masgrau A et al (2012) Time-course changes of muscle protein synthesis associated with obesity-induced lipotoxicity. J Physiol 590(Pt 20):5199–5210
Sitnick M, Bodine SC, Rutledge JC (2009) Chronic high fat feeding attenuates load-induced hypertrophy in mice. J Physiol 587(Pt 23):5753–5765
Bollheimer LC et al (2012) Sarcopenia in the aging high-fat fed rat: a pilot study for modeling sarcopenic obesity in rodents. Biogerontology 13(6):609–620
Rahman M et al (2009) Conjugated linoleic acid (CLA) prevents age-associated skeletal muscle loss. Biochem Biophys Res Commun 383(4):513–518
Murphy MP et al (1995) A simple and rapid test of sensorimotor function in the aged rat. Neurobiol Learn Mem 64(2):181–186
Murphy KT et al (2008) Antioxidant treatment with N-acetylcysteine regulates mammalian skeletal muscle Na+-K+-ATPase alpha gene expression during repeated contractions. Exp Physiol 93(12):1239–1248
Bonetto A et al (2011) STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS ONE 6(7):e22538
Adams GR (2006) Satellite cell proliferation and skeletal muscle hypertrophy. Appl Physiol Nutr Metab 31(6):782–790
Drummond MJ, Marcus RL, Lastayo PC (2012) Targeting anabolic impairment in response to resistance exercise in older adults with mobility impairments: potential mechanisms and rehabilitation approaches. J Aging Res 2012:486930
Phillips T, Leeuwenburgh C (2005) Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J 19(6):668–670
Suetta C et al (2012) Aging affects the transcriptional regulation of human skeletal muscle disuse atrophy. PLoS ONE 7(12):e51238
Siu PM, Alway SE (2005) Mitochondria-associated apoptotic signalling in denervated rat skeletal muscle. J Physiol 565(Pt 1):309–323
Budihardjo I et al (1999) Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 15:269–290
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281(5381):1312–1316
Alway SE, Siu PM (2008) Nuclear apoptosis contributes to sarcopenia. Exerc Sport Sci Rev 36(2):51–57
Acknowledgments
The authors acknowledge the efforts of Dr. Samuel C. Grant, Dr. Bahram H. Arjmandi, Dr. Paul C. Henning, and Dr. Chris Boehm for their technical assistance. This work was partially supported by Sekwang Inc., Florida State University, Vital Pharmaceuticals, and Ocean Nutrition.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, SR., Khamoui, A.V., Jo, E. et al. Effects of chronic high-fat feeding on skeletal muscle mass and function in middle-aged mice. Aging Clin Exp Res 27, 403–411 (2015). https://doi.org/10.1007/s40520-015-0316-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-015-0316-5