Abstract
Background
Neuroendocrine tumours (NETs) are a heterogeneous group of tumours that arise in different tissues and organs and have an endocrine and neurological interface that differentiates them into a stand-alone entity. One of their interesting and unique criteria is the overexpression of somatostatin receptors (SSRs) on their cell membrane, which has allowed for specific diagnostic imaging techniques and targeted therapy like peptide receptor radionuclide therapy (PRRT).
Objective
The aim of this study is to provide a literature review summarizing the latest available studies concerning the use of PRRT in treatment of neuroendocrine tumours including patient selection, the choice of PRPP, efficacy, side effects, and complications.
Methods
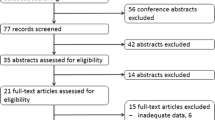
A comprehensive search strategy was used based on SCOPUS and PubMed databases. We considered all studies published in English evaluating the use of PRRT (177Lu-Dotatate and 90Y-Dotatate) in treatment of NETs and its effectiveness and side effects and complications.
Results
PRRT was found to be effective as monotherapy or in combination with other therapies. 90Yttrium may be more appropriate for larger tumour lesions, while 177Lutetium is more appropriately used for smaller ones. A combination therapy with 90Yttrium and 177Lutetium has been suggested for variable sized lesions. Mild acute side effects were reported more in 177Lutetium, while sub-acute and long-term side effects are more with 90Yttrium. Heamatotoxicity is usually mild and reversible and only < 15% may progress into severe toxicity. Renal toxicity was greatly reduced to < 3% by kidney protective measures.
Conclusions
PRRT is well-tolerated and effective treatment modality for non-operable and/or metastatic neuroendocrine tumours. Side effects are usually mild and reversible. More work needs to be done regarding standardization of dosing, timing, and patient selection criteria and ways of follow-up to obtain the maximum potential benefit.




Similar content being viewed by others
References
Körner M (2016) Specific biology of neuroendocrine tumors: peptide receptors as molecular targets. Best Pract Res Clin Endocrinol Metab 30(1):19–31
Public Health England (2016) Incidence and survival in neuroendocrine tumours and neuroendocrine carcinomas (NETs/NECs) in England, 2013–2014, PHE publications gateway number: 2016360
Kim JY, Hong S, Ro JY (2017) Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol 2017(29):11–16
Capella C, Heitz PU, Hofler H, Solcia E, Kloppel G (1995) Revised classification of neuroendocrine tumours of the lung, pancreas and gut. Virchows Archiv 425(6):547–560
Kim JY, Hong S (2016) Recent updates on neuroendocrine tumors from the gastrointestinal and pancreatobiliary tracts. Arch Pathol Lab Med 140(5):437–448
Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S (2010) The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 39(6):707–712
Aktolun C, Goldsmith SJ (2015) Nuclear oncology. Wolters Kluwer, Philadelphia
Pelosi G, Sonzogni A, Harari S et al (2017) Classification of pulmonary neuroendocrine tumors: new insights. Transl Lung Cancer Res. 6(5):513–529. https://doi.org/10.21037/tlcr.2017.09.04
Schnabel PA, Junker K (2015) Pulmonary neuroendocrine tumors in the new WHO 2015 classification: start of breaking new grounds? Pathologe. 36(3):283–292
Jian S (2017) Pancreatic neuroendocrine tumors. Intractable Rare Dis Res 6(1):21–28
Wcoazec JY, Couvelard A (2017) Classification of pancreatic neuroendocrine tumours: changes made in the 2017 WHO classification of tumours of endocrine organs and perspectives for the future. Ann Pathol 37(6):444–456
Reubi JC, Schar JC, Waser B, Wenger S, Heppeler A, Schmitt JS et al (2000) Affinity profiles for human somatostatin receptor subtypes SST1–SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med 27(3):273–282
Umberto G, Diego F, Marilena S, Renato S, Patrizia D, Jean-Luis R et al (2008) Treatment of a pituitary metastasis from a neuroendocrine tumour: case report and literature review. Pituitary 11:93–102
Scholzen T, Gerdes J (2010) The Ki-67 protein: from the known and the unknown. J Cell Physiol (Wiley-Liss, Inc.) 182(3):311–322
Mestier LD, Dromain C, Dassignies G et al (2013) Evaluating digestive neuroendocrine tumor progression and therapeutic responses in the era of targeted therapies: state of the art. Endocr Relat Cancer 21(3):R105-20
Berardi R, Rinaldi S, Torniai M, Morgese F, Partelli S, Caramanti M et al (2016) Gastrointestinal neuroendocrine tumors: searching the optimal treatment strategy—a literature review. Crit Rev Oncol Hematol 2016(98):264–274
Caplin ME, Pavel M, Cwikla JB, Phan AT, Raderer M, Sedlackova E et al (2014) Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 371(3):224–233
Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E et al (2011) Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 364(6):514–523
Mojtahedi A, Thamake S, Tworowska I et al (2014) The value of (68)Ga-DOTATATE PET/CT in diagnosis and management of neuroendocrine tumors compared to current FDA approved imaging modalities: a review of literature. Am J Nucl Med Mol Imaging 4:426e34
Toumpanakis C, Kim MK, Rinke A et al (2014) Combination of crosssectional and molecular imaging studies in the localization of gastroenteropancreatic neuroendocrine tumors. Neuroendocrinology 99:63e74
van Vliet EI, van Eijck CH, de Krijger RR et al (2015) Neoadjuvant treatment of nonfunctioning pancreatic neuroendocrine tumors with [177Lu-DOTA0, Tyr3] octreotate. J Nucl Med. https://doi.org/10.2967/jnumed.115.158899
Stoeltzing O, Loss M, Huber E et al (2010) Staged surgery with neoadjuvant 90Y-DOTATOC therapy for down-sizing synchronous bilobular hepatic metastases from a neuroendocrine pancreatic tumor. Langenbecks Arch Surg 395:185e92
Anthony LB, Woltering EA, Espenan GD, Cronin MD, Maloney TJ, McCarthy KE (2017) Indium-111-pentetreotide prolongs survival in gastroenteropancreatic malignancies. Semin Nucl Med 32(2):123–132
Valkema R, de Jong M, Bakker WH, Breeman WAP, Kooij PPM, Lugtenburg PJ et al (2002) Phase I study of peptide receptor radionuclide therapy with 111In-DTPA]octreotide: the rotterdam experience. Semin Nucl Med 32(2):110–122
van der Zwan WA, Bodei L, Mueller-Brand J, de Herder WW, Kvols LK, Kwekkeboom DJ (2015) GEPNETs update: radionuclide therapy in neuroendocrine tumors. Eur J Endocrinol 172(1):R1–R8
Romer A, Seiler D, Marincek N et al (2014) Somatostatin-based radiopeptide therapy with [177Lu-DOTA]-TOC versus [90Y-DOTA]-TOC in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 41:214e22
Villard L, Romer A, Marincek N et al (2012) Cohort study of somatostatin-based radiopeptide therapy with [90Y-DOTA]-TOC versus [90Y-DOTA]-TOC plus [177Lu-DOTA]-TOC in neuroendocrine cancers. J Clin Oncol 30:1100e6
Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H et al (2011) response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. JCO 29(17):2416–2423 (2017)
Valkema R, Pauwels S, Kvols LK, Barone R, Jamar F, Bakker WH et al (2006) Survival and response after peptide receptor radionuclide therapy with [90Y-DOTA0, Tyr3] octreotide in patients with advanced gastroenteropancreatic neuroendocrine tumors. Semin Nucl Med 36(2):147–156
Bushnell DL, O’Dorisio TM, O’Dorisio MS, Menda Y, Hicks RJ, Van Cutsem E et al (2010) 90Y-edotreotide for metastatic carcinoid refractory to octreotide. JCO 28(10):1652–1659 (2017)
Pfeifer AK, Gregersen T, Gronbaekk H, Hansen CP, Muller-Brand J, Herskind Bruun K et al (2011) Peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATOC in advanced neuroendocrine tumors: results from a Danish cohort treated in Switzerland. Neuroendocrinology 93(3):189–196
Cwikla JB, Sankowski A, Seklecka N, Buscombe JR, Nasierowska-Guttmejer A, Jeziorski KG et al (2010) Efficacy of radionuclide treatment DOTATATE Y-90 in patients with progressive metastatic gastroenteropancreatic neuroendocrine carcinomas (GEP-NETs): a phase II study. Ann Oncol 21(4):787–794
Bodei L, Cremonesi M, Grana CM, Fazio N, Iodice S, Baio SM et al (2011) Peptide receptor radionuclide therapy with 177Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging 38(12):2125–2135
Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP et al (2008) Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0, Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol 26(13):2124–2130
Neychev V, Kebebew E (2017) management options for advanced low or intermediate grade gastroenteropancreatic neuroendocrine tumors: review of recent literature. Int J Surg Oncol 2017:6424812
Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B et al (2017) Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med 376(2):125–135
Tessa B, Jaap T, Casper V, Gaston F, Richard F, Wouter D et al (2016) Peptide receptor radionuclide therapy of neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab 30:103e114
De Keizer B, van Aken MO, Feelders RA et al (2008) Hormonal crises following receptor radionuclide therapy with the radiolabeled somatostatin analogue [177Lu-DOTA0, Tyr3]octreotate. Eur J Nucl Med Mol Imaging 35:749e55
Kwekkeboom DJ, de Herder WW, Kam BL et al (2008) Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0, Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol 26:2124e30
Svensson J, Berg G, Wängberg B et al (2015) Renal function affects absorbed dose to the kidneys and haematological toxicity during 177Lu-DOTATATE treatment. Eur J Nucl Med Mol Imaging 42(6):947–955
Bodei L, Kidd M, Paganelli G et al (2015) Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging 42(1):5–19
Sierra ML, Agazzi A, Bodei L et al (2009) Lymphocytic toxicity in patients after peptide-receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE and 90Y-DOTATOC. Cancer Biother Radiopharm 24(6):659–665
Melis M, Krenning EP, Bernard BF, Barone R, Visser TJ, de Jong M (2005) Localisation and mechanism of renal retention of radiolabelled somatostatin analogues. Eur J Nucl Med Mol Imaging 32(10):1136–1143
Rolleman EJ, Valkema R, de Jong M, Kooij PP, Krenning EP (2003) Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur J Nucl Med Mol Imaging 30(1):9–15
Bergsma H, Konijnenberg MW, van der Zwan WA, Kam BLR, Teunissen JJM, Kooij PP et al (2016) Nephrotoxicity after PRRT with 177Lu-DOTA-octreotate. Eur J Nucl Med Mol Imaging 43(10):1802–1811
Kwekkeboom DJ, Krenning EP, Scheidhauer K, Lewington V, Lebtahi R, Grossman A et al (2009) ENETS consensus guidelines for the standards of care in neuroendocrine tumors: somatostatin receptor imaging with (111)In-pentetreotide. Neuroendocrinology 90(2):184–189
Valkema R, Pauwels SA, Kvols LK, Kwekkeboom DJ, Jamar F, de Jong M et al (2005) Long-term follow-up of renal function after peptide receptor radiation therapy with (90)Y-DOTA(0), Tyr(3)-octreotide and (177)Lu-DOTA(0), Tyr(3)-octreotate. J Nucl Med 46(Suppl 1):83S–91S
Bodei L, Cremonesi M, Ferrari M, Pacifici M, Grana CM, Bartolomei M et al (2008) Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging 35(10):1847–1856
Bodei L, Cremonesi M, Ferrari M et al (2008) Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging 35:1847e56
Zaknun JJ, Bodei L, Mueller-Brand J, Pavel ME, Baum RP, Horsch D et al (2013) The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 40(5):800–816
O’Toole D, Kianmanesh R, Caplin M (2016) ENETS 2016 consensus guidelines for the management of patients with digestive neuroendocrine tumors: an update. Neuroendocrinology 103(2):117–118
Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M et al (2016) ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 103(2):153–171
Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C et al (2007) 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med 48(4):508–518
Shi C, Gonzalez RS, Zhao Z, Koyama T, Cornish TC, Hande KR et al (2015) Liver metastases of small intestine neuroendocrine tumors: Ki67 heterogeneity and WHO grade discordance with primary tumors. Am J Clin Pathol 143(3):398–404
Zen Y, Heaton N (2013) Elevated Ki-67 labeling index in ‘synchronous liver metastases’ of well differentiated enteropancreatic neuroendocrine tumor. Pathol Int 63(11):532–538
Ezziddin S, Adler L, Sabet A, Poppel TD, Grabellus F, Yuce A et al (2014) Prognostic stratification of metastatic gastroenteropancreatic neuroendocrine neoplasms by 18F-FDG PET: feasibility of a metabolic grading system. J Nucl Med 55(8):1260–1266
Hindi E (2017) The NETPET score: combining FDG and somatostatin receptor imaging for optimal management of patients with metastatic well-differentiated neuroendocrine tumors. Theranostics 7(5):1159–1163
Ginj M, Zhang H, Waser B, Cescato R, Wild D, Wang X et al (2006) Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc Natl Acad Sci USA 103(44):16436–16441
Wild D, Fani M, Behe M, Brink I, Rivier JE, Reubi JC et al (2011) First clinical evidence that imaging with somatostatin receptor antagonists is feasible. J Nucl Med 52(9):1412–1417
Fani M, Nicolas GP, Wild D (2017) somatostatin receptor antagonists for imaging and therapy. J Nucl Med 58(Suppl 2):61S–66S
Fani M, Peitl PK, Velikyan I (2017) Current status of radiopharmaceuticals for the theranostics of neuroendocrine neoplasms. Pharmaceuticals. https://doi.org/10.3390/ph10010030
Reubi JC, Waser B, Macke H, Rivier J (2017) Highly increased 125I-JR11 antagonist binding in vitro reveals novel indications for sst2 targeting in human cancers. J Nucl Med 58(2):300–306
Fani M, Nicolas GP, Wild D (2017) Somatostatin receptor antagonists for imaging and therapy. J Nucl Med 58(Suppl 2):61S–66S
Marciniak A, Brasu J (2016) Somatostatin analogues labeled with copper radioisotopes: current status. J Radioanal Nucl 313(2):279–289
Chan HS, de Blois E, Morgenstern A, Bruchertseifer F, de Jong M, Breeman W et al (2017) In Vitro comparison of (213)Bi- and (177)Lu-radiation for peptide receptor radionuclide therapy. PLoS ONE 12(7):e0181473
Kratochwil C, Giesel FL, Bruchertseifer F, Mier W, Apostolidis C, Boll R et al (2014) (213)Bi-DOTATOC receptor-targeted alpha-radionuclide therapy induces remission in neuroendocrine tumours refractory to beta radiation: a first-in-human experience. Eur J Nucl Med Mol Imaging 41(11):2106–2119
Funding
The authors declare that there have been no financial contributions to this article.
Author information
Authors and Affiliations
Contributions
SY: content planning, literature search and review, manuscript writing, and editing. SA: literature search and review, manuscript writing, and editing. AA-N: content planning, literature search and review, manuscript writing, and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors, Siraj Yusuf, Shahad Alsadik, and Adil AL-Nahhas, have no conflict of interest to declare.
Ethical standards
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Rights and permissions
About this article
Cite this article
Yusuf, S., Alsadik, S. & AL-Nahhas, A. Peptide receptor radionuclide therapy for neuroendocrine tumours. Clin Transl Imaging 6, 101–111 (2018). https://doi.org/10.1007/s40336-018-0267-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-018-0267-x