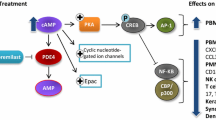
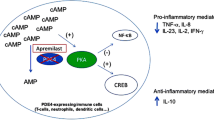
Abstract
Apremilast (Otezla®) is an oral phosphodiesterase 4 inhibitor indicated for the twice-daily treatment of adults with psoriasis and psoriatic arthritis (PsA). Its use in these patient populations has been assessed in two phase III clinical trial programmes (ESTEEM and PALACE). At 16 weeks in the two ESTEEM trials, apremilast reduced the severity and extent of moderate to severe plaque psoriasis, including nail, scalp and palmoplantar manifestations, versus placebo in adults, with these benefits generally being sustained over 52 weeks of treatment. Similarly, in three PALACE trials (PALACE 1–3), apremilast improved the signs and symptoms of PsA relative to placebo at 16 weeks in adults with active disease despite treatment with conventional synthetic and/or biologic disease-modifying anti-rheumatic drugs. These PsA benefits were generally sustained for up to 104 weeks of treatment; skin involvement, enthesitis and dactylitis also improved with the drug. Apremilast was generally well tolerated, with the most common adverse events being diarrhoea and nausea in the first year of treatment (usually occurring in the first 2 weeks after the first dose and resolving within 4 weeks) and nasopharyngitis and upper respiratory tract infection with continued treatment. Although further longer-term and comparative efficacy and tolerability data would be beneficial, the current clinical data indicate that apremilast is an effective and well tolerated option for the management of psoriasis and PsA in adults.



Similar content being viewed by others
References
National Institute for Health and Clinical Excellence. Psoriasis: the assessment and management of psoriasis. 2012. https://www.nice.org.uk/guidance/cg153. Accessed 26 June 2015.
National Psoriasis Foundation. Psoriasis and psoriatic arthritis. 2015. https://www.psoriasis.org. Accessed 26 June 2015.
The Arthritis Society. Psoriatic arthritis. 2015. http://www.arthritis.ca/page.aspx?pid=1011. Accessed 26 June 2015.
Gan EY, Chong WS, Tey HL. Therapeutic strategies in psoriasis patients with psoriatic arthritis: focus on new agents. BioDrugs. 2013;27(4):359–73.
Gossec L, Smolen JS, Gaujoux-Viala C, et al. European League Against Rheumatism recommendations for the management of psoriatic arthritis with pharmacological therapies. Ann Rheum Dis. 2012;71(1):4–12.
Coates LC, Tillett W, Chandler D, et al. The 2012 BSR and BHPR guideline for the treatment of psoriatic arthritis with biologics. Rheumatology. 2013;52(10):1754–7.
Celgene Europe Ltd. Otezla film-coated tablets: EU summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 8 July 2015.
Celgene Corporation. Otezla (apremilast) tablets, for oral use: US prescribing information. 2014. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed 8 July 2015.
Schafer PH, Parton A, Capone L, et al. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26(9):2016–29.
Schafer PH, Parton A, Gandhi AK, et al. Apremilast, a cAMP phosphodiesterase-4 inhibitor, demonstrates anti-inflammatory activity in vitro and in a model of psoriasis. Br J Pharmacol. 2010;159(4):842–55.
Gottlieb AB, Matheson RT, Menter A, et al. Efficacy, tolerability, and pharmacodynamics of apremilast in recalcitrant plaque psoriasis: a phase II open-label study. J Drugs Dermatol. 2013;12(8):888–97.
Gottlieb AB, Strober B, Krueger JG, et al. An open-label, single-arm pilot study in patients with severe plaque-type psoriasis treated with an oral anti-inflammatory agent, apremilast. Curr Med Res Opin. 2008;24(5):1529–38.
Schafer PH, Chen P, Fang L, et al. The pharmacodynamic impact of apremilast, an oral phosphodiesterase 4 inhibitor, on circulating levels of inflammatory biomarkers in patients with psoriatic arthritis: substudy results from a phase III, randomized, placebo-controlled trial (PALACE 1). J Immunol Res. 2015;2015:906349.
Papp K, Cather JC, Rosoph L, et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet. 2012;380(9843):738–46.
Schett G, Wollenhaupt J, Papp K, et al. Oral apremilast in the treatment of active psoriatic arthritis: results of a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2012;64(10):3156–67.
Papp KA, Kaufmann R, Thaçi D, et al. Efficacy and safety of apremilast in subjects with moderate to severe plaque psoriasis: results from a phase II, multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison study. J Eur Acad Dermatol Venereol. 2013;27(3):e376–83.
Hoffmann M, Kumar G, Schafer P, et al. Disposition, metabolism and mass balance of [(14)C]apremilast following oral administration. Xenobiotica. 2011;41(12):1063–75.
Wu A, Rohane P, Ng J, et al. Safety/tolerability and pharmacokinetics of multiple oral doses of apremilast in healthy male subjects [abstract no. PI-51]. In: 113th Annual Meeting of the American Society for Clinical Pharmacology and Therapeutics; 2012.
Liu Y, Kassir N, Mouksassi S, et al. Population pharmacokinetic and exposure-response analyses of apremilast in subjects with active psoriatic arthritis [abstract no. M-027]. J Pharmacokinet Pharmacodyn. 2013;40(1 Suppl 1):S34–S5.
Liu Y, Zhou S, Nissel J, et al. The pharmacokinetic effect of coadministration of apremilast and methotrexate in individuals with rheumatoid arthritis and psoriatic arthritis. Clin Pharmacol Drug Devel. 2014;3(6):456–65.
Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73(6):1020–6.
Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Longterm (52-week) results of a phase III randomized, controlled trial of apremilast in patients with psoriatic arthritis. J Rheumatol. 2015;42(3):479–88.
Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73(1):37–49.
US National Institutes of Health. Clinicaltrials.gov identifiers: NCT01194219 and NCT01232283. 2015. http://www.clinicaltrials.gov. Accessed 23 Jan 2015.
Paul C, Crowley J, Cather J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe psoriasis: 16-week results of a phase 3, randomized, controlled trial (ESTEEM 2) [abstract no. P8412]. J Am Acad Dermatol. 2014;70(5 Supp 1):AB164.
Papp K, Griffiths C, Leonardi C, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe psoriasis: results from the randomized treatment withdrawal phase of a phase 3, randomized, controlled trial (ESTEEM 1) [abstract no. P8359]. J Am Acad Dermatol. 2014;70(5 Supp 1):AB164.
Feldman S, Thaci D, Ling M, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis: pruritus and DLQI correlations at week 16 (ESTEEM 1 and 2) [abstract no. 1092]. J Am Acad Dermatol. 2015;72(5 Suppl 1):AB226.
Bissonnette R, Cather J, Rich P, et al. Efficacy of apremilast, an oral phosphodiesteras 4 inhibitor, for palmoplantar psoriasis in patients with moderate to severe plaque psoriasis in phase 2 and 3 (ESTEEM) trials [abstract no. 1131]. J Am Acad Dermatol. 2015;72(5 Suppl 1):AB234.2015.
Kuznik A, Li S, Wu J. Estimating the proportion of patients who achieve a clinically meaningful benefit from apremilast therapy in the ESTEEM trials [abstract no. 1190]. J Am Acad Dermatol. 2015;72(5 Suppl 1):AB235.
Papp K, Zhang F, Tencer T, et al. The impact of apremilast therapy on work productivity among patients with moderate to severe plaque psoriasis: pooled analysis of 2 phase 3 studies [abstract no. 1151]. J Am Acad Dermatol. 2015;72(5 Suppl 1):AB255.
Papp K, Crowley J, Paul C, et al. Apremilast, an oral phosphodiesterase 4 inhibitor: improvements in nail and scalp psoriasis and psoriasis area and severity index in patients with moderate to severe plaque psoriasis (ESTEEM 1 and 2) [abstract no. THU0413]. In: 16th Annual EULAR Congress; 2015.
Crowley J, Gooderham M, Wasel N, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with nail, scalp, and palmoplantar psoriasis: 52-week results from the ESTEEM 2 study [abstract no. 894]. J Am Acad Dermatol. 2015;72(5 Suppl 1):AB226.
US National Institutes of Health. Clinicaltrial.gov identifiers: NCT01172938, NCT01212757 and NCT01212770. 2015. http://www.clinicaltrials.gov. Accessed 23 Jan 2015.
Cutolo M, Myerson GE, Fleischmann RM, et al. Long-term (52-week) results of a phase 3, randomized, controlled trial of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis (PALACE 2) [abstract no. 815]. In: 77th American College of Rheumatology and 48th Association of Rheumatology and Health Professionals Annual Meeting; 2013.
Birbara C, Blanco FJ, Crowley JJ, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis including current skin involvement: results of a phase 3, randomized, controlled trial [abstract no. OP0104]. In: 14th Annual EULAR Congress; 2013.
Schett G, Mease P, Gladman D, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, and the impact of baseline weight and body mass index (BMI) on ACR20 and HAQ-DI response: pooled results from 3 phase 3, randomized, controlled trials [abstract no. AB0746]. In: 15th Annual EULAR Congress; 2014.
Hall S, Cutolo M, Mease PJ, et al. Tender and swollen joint count improvement with apremilast [abstract no. APLAR-0289]. Int J Rheum Dis. 2014;17(Suppl 1):80.
Kavanaugh A, Cutolo M, Mease P, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, is associated with long-term (52-week) improvement in measures of disease activity in patients with psoriatic arthritis: results from 3 phase 3, randomized, controlled trials [abstract no. OP0078]. Ann Rheum Dis. 2014;73(Suppl 2):90–1.
Gladman DD, Strand V, Kavanaugh A, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, is associated with improvement of pain, fatigue, and disability in patients with psoriatic arthritis: results from 3 phase 3, randomized, controlled trials. [abstract no. 1565]. Arthritis Rheumatol. 2014;66 Supp(10):S691.
Zhang F, Tencer T, Li S. Work productivity improvement associated with apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis: results of a phase 3, randomized, controlled trial [abstract no. SAT0392]. In: 15th Annual EULAR Congress; 2014.
Edwards CJ, Blanco FJ, Crowley J, et al. Efficacy of apremilast, an oral phosphodiesterase 4 inhibitor, on physical function and pain in patients with psoriatic arthritis and current skin involvement: results of palace 3, a phase 3, randomized, controlled trial [abstract no. 214]. Rheumatology. 2014;53(Suppl 1):i139–40.
Schett G, Mease PJ, Gladman DD, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, is associated with long-term (52-week) improvement in physical function in patients with psoriatic arthritis: results from three phase 3, randomized, controlled trials [abstract no. 331]. In: 77th American College of Rheumatology and 48th Association of Rheumatology and Health Professionals Annual Meeting; 2013.
Bird P, Blanco F, Kavanaugh A, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis: results of phase 3, randomized, placebo-controlled trials (PALACE 1, 2, and 3) [abstract no. ARA-P34]. Intern Med J. 2014;44(Suppl 2):19.
Zhang F, Clancy Z, Li S. Long-term work productivity improvement associated with apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis: pooled analysis of 3 phase 3 studies. In: 16th Annual EULAR Congress; 2015.
Gladman D, Mease P, Kavanaugh A, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, is associated with long-term (52-week) improvements in enthesitis and dactylitis in patients with psoriatic arthritis: pooled results from three phase 3, randomized, controlled trials [abstract no. 13]. J Rheumatol. 2014;41(7):1448.
Kavanaugh A, Adebajo A, Gladman D, et al. Long-term (104-week) efficacy and safety profile of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis: results from a phase III, randomized, controlled trial and open-label extension (PALACE 1) [abstract no. THU0420]. In: 16th Annual EULAR Congress; 2015.
Zhang F, Clancy Z, Li S, et al. Long-term impact of apremilast on physical function in patients with psoriatic arthritis using the HAQ-DI assessment [abstract no. AB0805]. In: 16th Annual EULAR Congress; 2015.
Edwards C, Blanco F, Crowley J, et al. Disease activity and safety during long-term (104-week) treatment with apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis: results from a phase III, randomized, controlled trial and open-label extension (PALACE 3) [abstract no. THU0416]. In: 16th Annual EULAR Congress; 2015.
Gladman D, Kavanaugh A, Adebajo A, et al. Apremilast, an oral phophodiesterase 4 inhibitor, is associated with long-term (104-week) improvements in enthesitis and dactylitis in patients with psoriatic arthritis: pooled results from three phase 3, randomized, controlled trials [abstract no. OP0169]. In: 16th Annual EULAR Congress; 2015.
Mease P, Adebajo A, Gladman D, et al. Long-term (104-week) safety profile of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis: pooled safety analysis of three phase 3, randomized, controlled trials [abstract THU0432]. In: 16th Annual EULAR Congress; 2015.
Papp KA, Reich K, Sobell J, et al. Two-year safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis: results from a phase 3, randomized, controlled trial (ESTEEM 1) [abstract no. 1055]. J Am Acad Derm. 2015;72(5 Suppl 1):AB256.
Reich K, Sobell J, Stevens R, et al. Change in weight with apremilast, an oral phosphodiesterase 4 inhibitor: pooled analysis of the ESTEEM 1 and ESTEEM 2 trials [abstract no. 1162]. J Am Acad Dermatol. 2015;72(5 Suppl 1):AB227.
Mease P, Gladman D, Kavanaugh A, et al. Change in weight from baseline during the PALACE clinical trial program with apremilast, an oral phosphodiesterase 4 inhibitor: pooled results from 3 phase 3, randomized, controlled trials [abstract no. AB0758]. In: 15th Annual EULAR Congress; 2014.
The British Society for Rheumatology and British Health. Professionals in Rheumatology. The 2012 BSR and BHPR guideline for the treatment of psoriatic arthritis with biologics. Rheumatology. 2012;52(10):1754–7.
Mease PJ. Apremilast: a phosphodiesterase 4 inhibitor for the treatment of psoriatic arthritis. Rheumatol Ther. 2014;1(1):1–20.
Matucci A, Petroni G, Nencini F, et al. Anti-drug antibodies and clinical implications. Clin Dermatol. 2013;1(2):77–80.
Papoutsaki M, Costanzo A. Treatment of psoriasis and psoriatic arthritis. BioDrugs. 2013;27(Suppl 1):3–12.
Novartis Europharm Limited. Cosentyx for injection: EU summary of product characteristics. 2015. http://www.ema.europa.eu. Accessed 8 July 2015.
Novartis. CosentyxTM (secukinumab) injection, for subcutaneous use: US prescribing information. 2015. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm. Accessed 8 July 2015.
Wells A, Adebajo AO, Aelion JA, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, is associated with long-term (52-week) improvement in the signs and symptoms of psoriatic arthritis in DMARD-naive patients: results from a phase 3, randomized, controlled trial. [abstract no. 1543]. Arthritis Rheumatol. 2014;66 Supp.(10):S680.
Armstrong AW, Betts K, Sundaram M, et al. Comparative efficacy of methotrexate versus apremilast for methotrexate-naïve psoriasis patients: an indirect comparison [abstract no. 1278]. J Am Acad Dermatol. 2015;72(5 Suppl 1);AB229.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit. Emma Deeks is a salaried employee of Adis/Springer.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: G. Han, Division of Dermatology, Albert Einstein College of Medicine, New York, NY, USA; I. Olivieri, Rheumatology Department of Lucania, San Carlo Hospital of Potenza and Madonna delle Grazie Hospital of Matera, Potenza, Italy; M. Papoutsaki, University Department of Dermatology, A. Sygros Hospital, Athens, Greece.
Rights and permissions
About this article
Cite this article
Deeks, E.D. Apremilast: A Review in Psoriasis and Psoriatic Arthritis. Drugs 75, 1393–1403 (2015). https://doi.org/10.1007/s40265-015-0439-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0439-1