Abstract
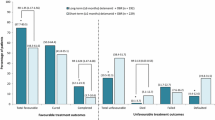
Delamanid (Deltyba®), a nitroimidazo-oxazole derivative, is a new anti-tuberculosis (TB) drug which exhibits potent in vitro and in vivo antitubercular activity against drug-susceptible and -resistant strains of Mycobacterium tuberculosis. It is approved in several countries, including Japan and those of the EU, for use as part of an appropriate combination regimen in adults with multidrug-resistant tuberculosis (MDR-TB) when an effective treatment regimen cannot otherwise be composed due to resistance or tolerability. In a robust phase II trial in adult patients with MDR-TB, oral delamanid 100 mg twice daily for 2 months plus an optimized background regimen improved sputum culture conversion rates to a significantly greater extent than placebo. In a 6-month extension study, long-term (≤8 months) treatment with delamanid was associated with a higher incidence of favourable outcomes (i.e. cured or completed all treatment) than short-term (≤2 months) treatment, with an accompanying reduction inunfavourable outcomes as defined by the WHO (i.e. pre-specified proportion of TB-positive sputum cultures, death or treatment discontinuation for ≥2 months without medical approval). Delamanid was not associated with clinically relevant drug-drug interactions, including with antiretroviral drugs and those commonly used in treating TB. Delamanid was generally well tolerated in patients with MDR-TB, with gastrointestinal adverse events and insomnia reported most commonly. Although the incidence of QT interval prolongation was higher with delamanid-based therapy, it was not associated with clinical symptoms such as syncope and arrhythmia. In conclusion, delamanid is a useful addition to the treatment options currently available for patients with MDR-TB.



Similar content being viewed by others
References
World Health Organization. Global tuberculosis report 2013. 2013. http://www.who.int/tb/publications/global_report/en/. Accessed 7 Nov 2014.
Lange C, Abubakar I, Alffenaar JW, et al. Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement. Eur Respir J. 2014;44(1):23–63.
Zumla AI, Gillespie SH, Hoelscher M, et al. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet Infect Dis. 2014;14(4):327–40.
Brigden G, Nyang’wa B-T, du Cros P, et al. Principles for designing future regimens for multidrug-resistant tuberculosis. Bull World Health Organ. 2014;92(1):68–74.
European Medicines Agency. Delamanid assessment report. 2013. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002552/human_med_001699.jsp&mid=WC0b01ac058001d124. Accessed 7 Nov 2014.
Matteelli A, Roggi A, Carvalho ACC. Extensively drug-resistant tuberculosis: epidemiology and management. Clin Epidemiol. 2014;6(1):111–8.
Dooley KE, Nuermberger EL, Diacon AH. Pipeline of drugs for related diseases: tuberculosis. Curr Opin HIV AIDS. 2013;8(6):579–85.
Kwon Y-S, Jeong B-H, Koh W-J. Tuberculosis: clinical trials and new drug regimens. Curr Opin Pulm Med. 2014;20(3):280–6.
Reviriego C. Delamanid antimycobacterial agent treatment of tuberculosis. Drug Future. 2013;38(1):7–12.
Field SK. Safety and efficacy of delamanid in the treatment of multidrug-resistant tuberculosis (MDR-TB). Clin Med Insights Ther. 2013;5:137–49.
European Medicines Agency. Delamanid (Deltyba): summary of product characteristics. 2014. http://ec.europa.eu/health/documents/community-register/2014/20140428126881/anx_126881_en.pdf. Accessed 7 Nov 2014.
Diacon AH, Von Groote-Bidlingmaier F, Donald PR. Delamanid, a new 6-nitro-2,3-dihydroimidazo[2,1-b]oxazole for the management of tuberculosis resistant to at least isoniazid and rifampicin. Expert Opin Orphan Drugs. 2014;2(1):87–94.
Hurdle JG, Lee RB, Budha NR, et al. A microbiological assessment of novel nitrofuranylamides as anti-tuberculosis agents. J Antimicrob Chemother. 2008;62(5):1037–45.
Matsumoto M, Hashizume H, Tomishige T, et al. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 2006;3(11):e466.
van den Boogaard J, Kibiki GS, Kisanga ER, et al. New drugs against tuberculosis: problems, progress, and evaluation of agents in clinical development. Antimicrob Agents Chemother. 2009;53(3):849–62.
Saliu OY, Crismale C, Schwander SK, et al. Bactericidal activity of OPC-67683 against drug-tolerant Mycobacterium tuberculosis. J Antimicrob Chemother. 2007;60(5):994–8.
Diacon AH, Dawson R, Hanekom M, et al. Early bactericidal activity of delamanid (OPC-67683) in smear-positive pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 2011;15(7):949–54.
Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med. 2012;366(23):2151–60.
Otsuka Pharmaceutical Co. Ltd. Deltyba (delamanid): Japanese prescribing information. Tokyo: Otsuka Pharmaceutical Co., Ltd.; 2014.
Paccaly A, Petersen C, Patil S, et al. Absence of clinically relevant drug interaction between delamanid, a new drug for multidrug-resistant tuberculosis (MDR-TB) and tenofovir or lopinavir/ ritonavir in healthy subjects [abstract no. WEPE043]. In: 19th international AIDS conference. Washington, DC; 22–27 Jul 2012.
Petersen C, Paccaly A, Kim J, et al. Delamanid, a new drug for multi-drug resistant tuberculosis (MDR-TB), and efavirenz do not show clinically relevant drug interactions in healthy subjects [abstract no. A-1255]. In: 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy. San Fransisco; 9–12 Sep 2012.
Skripconoka V, Danilovits M, Pehme L, et al. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J. 2013;41(6):1393–400.
Zhang Q, Liu Y, Tang S, et al. Clinical benefit of delamanid (OPC-67683) in the treatment of multidrug-resistant tuberculosis patients in China. Cell Biochem Biophys. 2013;67(3):957–63.
Medecins Sans Frontieres. DR-TB drugs under the microscope: sources and prices for drug resistant TB medications. 2013. http://www.msfaccess.org/content/dr-tb-drugs-under-microscope3rd-edition. Accessed 7 Nov 2014.
Otsuka Pharmaceutical Development & Commercialization Inc. Safety and efficacy trial of delamanid for 6 months in patients with multidrug resistant tuberculosis [ClinicalTrials.gov identifier NCT01424670]. US National Institutes of Health, ClinicalTrials.gov. 2011. http://clinicaltrials.gov/show/NCT01424670. Accessed 7 Nov 2014.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the authors on the basis of scientific and editorial merit. Hannah Blair and Lesley Scott are salaried employees of Adis/Springer.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: S. K. Field, Division of Respirology, University of Calgary, Calgary, AB, Canada; D. Goletti, National Institute for Infectious Diseases “L. Spallanzani”, Rome, Italy; J-P. Zellweger, TB Competence Center, Swiss Lung Association, Berne, Switzerland; Q. Zhang, Tuberculosis Diagnosis and Treatment Center, Shanghai Pulmonary Hospital, Shanghai, China.
Rights and permissions
About this article
Cite this article
Blair, H.A., Scott, L.J. Delamanid: A Review of Its Use in Patients with Multidrug-Resistant Tuberculosis. Drugs 75, 91–100 (2015). https://doi.org/10.1007/s40265-014-0331-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-014-0331-4