Abstract
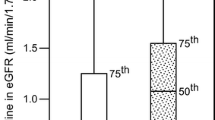
Predominantly diabetic nephropathy starts with glomerulosclerosis, when plasma molecules cross the dismantled glomerular basement membrane (GBM) and subsequently appear in urine. Therefore proteinuria is a sensitive criterion to diagnose progressive renal impairment and the presence of immunoglobulin like large molecules is potentially able to predict severity of nephropathy. Directly, or indirectly hyperglycemia induces proteinuria and high urinary excretion of IgG appears with progression of glomerular injury. This is an observational study of 683 patients with type 2 diabetes mellitus. Patients with varying degree of proteinuria were enrolled and classified into three groups according to the urinary albumin creatinine ratio (UACR, <29, 30–299, >300 mg/g creatinine.) and each group was further sub-classified into the low and high urinary IgG creatinine ratio (UIgGCR) based on the median value. Biochemical parameters were analyzed by standard laboratory methods. The association of proteinuria and odds ratio for risk factors of diabetic nephropathy was estimated using multinomial logistic regression models. The normoalbuminuric and microalbuminuric patients with high UIgGCR had shown lower eGFR (p < 0.05). There was no interaction observed between higher UIgGCR and lower eGFR in regression model analysis. Multinomial logistic regression model estimated the odds ratio for the AGEs, AOPP, lipid hydroperoxides and lipid peroxidation products were increased; 5.64 (95% CI 3.52–9.04), 1.03 (95% CI 1.02–1.04), 2.71 (95% CI 2.05–3.57) and 13.72 (95% CI 6.98–26.95) respectively with high UIgGCR in diabetic patients. High UIgGCR has shown a significant association with decreased eGFR and increased odds for potential hazardous factors. The current study has shown UIgGCR as an increased relative risk, and threat for rapid progression of diabetic nephropathy, possibly because GBM is the primary vulnerable site for deterioration by several hazardous metabolites. In conclusion, association between fractional clearance of IgG and relative risk of GBM threatening factors should be useful for prediction of progressive nature of diabetic nephropathy.



Similar content being viewed by others
References
Kitada M, Zhang Z, Mima A, King GL. Molecular mechanisms of diabetic vascular complications. J Diabetes Invest. 2010;1:77–89.
Tryggvason K, Wartiovaara J. How does the kidney filter plasma? Physiol. 2005;20:96–101.
Prato SD. Role of glucotoxicity and lipotoxicity in the pathophysiology of type 2 diabetes mellitus and emerging treatment strategies. Diabet Med. 2009;26:1185–92.
Miyata T, Fu MX, Kurokawa K, De-Strihou CVY, Thorpe SR, Baynes JW. Autoxidation products of both carbohydrates and lipids are increased in uremic plasma: Is there oxidative stress in uremia? Kidney Int. 1998;54:1290–5.
Fukasawa H, Bornheimer S, Kudlicka K, Farquhar MG. Slit diaphragms contain tight junction proteins. J Am Soc Nephrol. 2009;20:1491–503.
Sarav M, Wang Y, Hack BK, Chang A, Jensen M, Bao L, et al. Renal FcRn reclaims albumin but facilitates elimination of IgG. J Am Soc Nephrol. 2009;20:1941–52.
Thorner PS, Ho M, Eremina V, Sado Y, Quaggin S. Podocytes contribute to the formation of glomerular crescents. J Am Soc Nephrol. 2008;19:495–502.
Nagai R, Mori T, Yamamoto Y, Kaji Y, Yonei Y. Significance of advanced glycation end products in aging-related disease. Anti-Aging Med. 2010;7:112–9.
Kalousova M, Skrha J, Zima T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol Res. 2002;51:597–604.
Rabelink TJ, de Boer HC, van Zonneveld AJ. Endothelial activation and circulating markers of endothelial activation in kidney disease. Nat Rev Nephrol. 2010;6:404–14.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75.
Kroll MH, Chesler R, Hagengruber C, Blank DW, Kestner J, Rawe M. Automated determination of urinary creatinine without sample dilution: Theory and practice. Clin Chem. 1986;32:446–52.
Chandalia HB, Sadikot S, Bhargav DK, Krishnaswami PR. Estimation of glycosylated hemoglobins by a simple chemical method and its use in monitoring control of diabetes mellitus. J Assoc Phys India. 1980;28:285–6.
Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94.
Kakkar P, Das B, Vishwanathan PN. A modified spectrophtometeric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2.
Flohe L, Gunzler WA. Assay of glutathione peroxidase. Methods Enzymol. 1984;105:114–21.
Charlton-Menys V, Liu Y, Durrington NP. Semiautomated method for determination of serum paraoxonase activity using paraoxon as substrate. Clin Chem. 2006;52:453–7.
Mallikarjunappa S, Prakash M. Urine protein thiol in chronic renal failiour patients. Indian J Nephrol. 2007;17:7–9.
Benzin FFI, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxident Power”: the FRAP Assay. Anal Biochem. 1996;239:70–6.
Mashiba S, Uchida K, Okuda S, Tomitab S. Measurement of glycated albumin by the nitroblue tetrazolium calorimetric method. Clinica Chimica Acta. 1992;212:3–15.
Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–13.
Prakash M, Upadhya S, Prabhu R. Protein thiol oxidation and lipid peroxidation in patients with uraemia. Scand J Clin Lab Invest. 2004;64:599–604.
Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–5.
Mistry K, Kalia K. Non enzymatic glycosylatio of IgG and their urinary excretion in patients with diabetic nephropathy. Indian J Clin Biochem. 2008;23:159–65.
Singh NP, Ingle GK, Saini VK, Jami A, Beniwal P, Lal M, et al. Prevalence of low glomerular filtration rate, proteinuria and associated risk factors in north India using Cockcroft-Gault and modification of diet in renal disease equation: an observational, cross-sectional study. BMC Nephrol. 2009;10:1–13.
Forbes JM, Cooper ME, Oldfield MD, Thomas MC. Role of advanced glycation end product in diabetic nephropathy. J Am Nephrol. 2003;14:254–8.
Singh A, Satchell SC, Neal CR, McKenzie EA, Tooke JE, Mathieson PW. Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J Am Soc Nephrol. 2007;18:2885–93.
Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116:288–96.
Gupta A, Tripathi AK, Tripathi RL, Madhu SV, Banerjee BD. Advanced glycosylated end products-mediated activation of polymorphonuclear neutrophils in diabetes mellitus and associated oxidationve stress. Indian J Biochem Biophy. 2007;44:373–8.
Mehrotra S, Ling KLE, Belele Y, Gerbino E, Earle KA. Susceptibility in African- Caribbean and Caucasian patients with type 2 diabetes mellitus. Diabet Med. 2001;18:109–15.
Connelly PW, Zinman B, Maguire GF, Mamakeesick M, Harris SB, Hegele RA, et al. Association of the novel cardiovascular risk factors paraoxonase 1 and cystatin C in type 2 diabetes. J Lipid Res. 2009;50:1216–22.
Mastorikou M, Mackness B, Liu Y, Mackness M. Glycation of paraoxonase-1 inhibits its activity and impairs the ability of high-density lipoprotein to metabolize membrane lipid hydroperoxides. Diabet Med. 2008;25:1049–55.
Shi XY, Hou FF, Niu HX, Wang GB, Xie D, Guo ZJ, et al. Advanced oxidation protein products promote inflammation in diabetic kidney through activation of renal nicotinamide adenine dinucleotide phosphate oxidase. Endocrinol. 2008;149:1829–39.
Krzystek-Korpacka M, Salmonowicz B, Boehm D, Berdowska I, Zielinski B, Patryn E, et al. Diagnostic potential of oxidative stress markers in children and adolescents with type 1 diabetes. Clin Biochem. 2008;41:48–55.
Piwowar A, Knapik-Kordecka M, Warwas M. AOPP and its relations with selected markers of oxidative/antioxidative system in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2007;77:188–92.
Acknowledgement
We are thankful to Prof. Arvind Pandey (Director), and Dr. Tulsi Adhikari (Research Officer), from Institute for Research in Medical Statistics (ICMR), New Delhi India, for their assistance in statistical analysis and Dr Khan, Department of English, Sardar Patel University for revising the English language of the manuscript. We also acknowledge to GSBTM Gandhinagar, Gujarat and UGC New Delhi, India, to provide Financial assistance for the accomplishment of this work. We wish to sincerely thank all the patients for their cooperation during this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohan, S., Kalia, K. & Mannari, J. Diabetic nephropathy and associated risk factors for renal deterioration. Int J Diabetes Dev Ctries 32, 52–59 (2012). https://doi.org/10.1007/s13410-011-0047-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-011-0047-x