Abstract
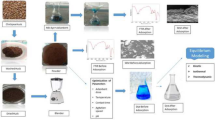
The methylene blue adsorption was carried out on a natural material powder of Acorus calamus treated firstly with H2SO4 and then activated by KMnO4. The new material was called PACK. Fourier transform infrared (FT-IR) spectroscopy, pHpzc analysis, and SEM micrograph were carried out to characterize the material. Pseudo-first-order, pseudo-second-order and pseudo-nth order constant rates were calculated for analysis of the dynamics of the sorption process, showing that sorption kinetics followed a pseudo-nth order model. Among the tested isotherm models, the R-P isotherm was considered to be the most relevant to describe MB sorption onto PACK. Advanced statistical physics models, monolayer single-energy, monolayer two-energy, and double-layer two-energy were used to analyze the adsorption mechanism of methylene blue (MB) and to understand the PACK adsorbent performance. Based on the R2 values obtained, the monolayer two-energy model was found to be the most suitable to describe the MB adsorption onto the PACK material. From this, the adsorption of MB was assumed on two different sites of PACK with two different energies, E1 for the first site and E2 for the second site. These two different receptor sites can interact with a variable number of MB molecules (n), n1 with the first type of sites and n2 with the second type of sites. The sorption capacity of this material was about 1500 mg/g at 30 °C. The potential of PACK, a readily available material to use as an alternative biosorbent material to eliminate the MB color from aqueous solutions, was therefore confirmed.











Similar content being viewed by others
References
Novel activated carbon prepared from an agricultural waste, Stipa tenacissima, based on ZnCl2 activation–characterization and application to the removal of methylene blue: Desalination and Water Treatment: Vol 57, No 50. https://www.tandfonline.com/doi/abs/10.1080/19443994.2015.1137231. Accessed 9 Mar 2020
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77:247–255. https://doi.org/10.1016/S0960-8524(00)00080-8
Soloman PA, Basha CA, Velan M, Ramamurthi V, Koteeswaran K, Balasubramanian N (2009) Electrochemical degradation of Remazol Black B dye effluent. Clean Soil Air Water 37:889–900. https://doi.org/10.1002/clen.200900055
Miyah Y, Lahrichi A, Idrissi M, Boujraf S, Taouda H, Zerrouq F (2017) Assessment of adsorption kinetics for removal potential of crystal violet dye from aqueous solutions using Moroccan pyrophyllite. J Assoc Arab Univ Basic Appl Sci 23:20–28. https://doi.org/10.1016/j.jaubas.2016.06.001
Juang RS, Wu FC, Tseng RL (1997) The ability of activated clay for the adsorption of dyes from aqueous solutions. Environ Technol 18:525–531. https://doi.org/10.1080/09593331808616568
Rangabhashiyam S, Anu N, Selvaraju N (2013) Sequestration of dye from textile industry wastewater using agricultural waste products as adsorbents. J Environ Chem Eng 1:629–641. https://doi.org/10.1016/j.jece.2013.07.014
Ahmed MJ, Dhedan SK (2012) Equilibrium isotherms and kinetics modeling of methylene blue adsorption on agricultural wastes-based activated carbons. Fluid Phase Equilib 317:9–14. https://doi.org/10.1016/j.fluid.2011.12.026
Şenol ZM (2020) Effective biosorption of Allura red dye from aqueous solutions by the dried-lichen (Pseudoevernia furfuracea) biomass. Int J Environ Anal Chem:1–15. https://doi.org/10.1080/03067319.2020.1785439
Badr S, Ashmawy AA, El Sherif I, Moghazy R (2016) Non-conventional low-cost biosorbents for adsorption and desorption of heavy metals. Res J Pharm, Biol Chem Sci 7:3110–3122
Berrios M, Martín MÁ, Martín A (2012) Treatment of pollutants in wastewater: adsorption of methylene blue onto olive-based activated carbon. J Ind Eng Chem 18:780–784. https://doi.org/10.1016/j.jiec.2011.11.125
Suárez-García F, Martínez-Alonso A, Tascón JMD (2001) Porous texture of activated carbons prepared by phosphoric acid activation of apple pulp. Carbon 39:1111–1115. https://doi.org/10.1016/S0008-6223(01)00053-7
Attia AA, Girgis BS, Fathy NA (2008) Removal of methylene blue by carbons derived from peach stones by H3PO4 activation: batch and column studies. Dyes Pigments 76:282–289. https://doi.org/10.1016/j.dyepig.2006.08.039
El-Hendawy A-NA, Samra SE, Girgis BS (2001) Adsorption characteristics of activated carbons obtained from corncobs. Colloids Surf A Physicochem Eng Asp 180:209–221. https://doi.org/10.1016/S0927-7757(00)00682-8
Baquero M (2003) Activated carbons by pyrolysis of coffee bean husks in presence of phosphoric acid. J Anal Appl Pyrolysis 70:779–784. https://doi.org/10.1016/S0165-2370(02)00180-8
Kyzas GZ, Lazaridis NK, Mitropoulos AC (2012) Removal of dyes from aqueous solutions with untreated coffee residues as potential low-cost adsorbents: equilibrium, reuse and thermodynamic approach. Chem Eng J 189–190:148–159. https://doi.org/10.1016/j.cej.2012.02.045
Namane A, Mekarzia A, Benrachedi K et al (2005) Determination of the adsorption capacity of activated carbon made from coffee grounds by chemical activation with ZnCl and HPO. J Hazard Mater 119:189–194. https://doi.org/10.1016/j.jhazmat.2004.12.006
Yagmur E, Ozmak M, Aktas Z (2008) A novel method for production of activated carbon from waste tea by chemical activation with microwave energy. Fuel 87:3278–3285. https://doi.org/10.1016/j.fuel.2008.05.005
Valix M, Cheung WH, McKay G (2004) Preparation of activated carbon using low temperature carbonisation and physical activation of high ash raw bagasse for acid dye adsorption. Chemosphere 56:493–501. https://doi.org/10.1016/j.chemosphere.2004.04.004
Laine J, Calafat A, Labady M (1989) Preparation and characterization of activated carbons from coconut shell impregnated with phosphoric acid. Carbon 27:191–195. https://doi.org/10.1016/0008-6223(89)90123-1
Önal Y (2006) Kinetics of adsorption of dyes from aqueous solution using activated carbon prepared from waste apricot. J Hazard Mater 137:1719–1728. https://doi.org/10.1016/j.jhazmat.2006.05.036
Patel R, Suresh S (2008) Kinetic and equilibrium studies on the biosorption of reactive black 5 dye by Aspergillus foetidus. Bioresour Technol 99:51–58. https://doi.org/10.1016/j.biortech.2006.12.003
Aksu Z (2005) Application of biosorption for the removal of organic pollutants: a review. Process Biochem 40:997–1026. https://doi.org/10.1016/j.procbio.2004.04.008
Dotto GL, Pinto LAA (2011) Adsorption of food dyes onto chitosan: optimization process and kinetic. Carbohydr Polym 84:231–238. https://doi.org/10.1016/j.carbpol.2010.11.028
Dotto GL, Vieira MLG, Esquerdo VM, Pinto LAA (2013) Equilibrium and thermodynamics of azo dyes biosorption onto Spirulina platensis. Braz J Chem Eng 30:13–21. https://doi.org/10.1590/S0104-66322013000100003
Kaur H, Thakur A (2014) Adsorption of Congo red dye from aqueous solution onto ash of Cassia fistula seeds: kinetic and thermodynamic studies. Chem Sci Rev Lett 3:0–169
Low cost adsorbents from agricultural waste for removal of dyes - Ramaraju - 2014 - Environmental Progress & Sustainable Energy - Wiley Online Library. https://aiche.onlinelibrary.wiley.com/doi/full/10.1002/ep.11742. Accessed 6 Nov 2020
Ramakrishnaiah C (2014) Removal of colour from textile effluent by adsorption using low cost adsorbents. IRJPAC 4:568–577. https://doi.org/10.9734/IRJPAC/2014/5772
Chebli D, Bouguettoucha A, Mekhalef T, Nacef S, Amrane A (2015) Valorization of an agricultural waste, Stipa tenassicima fibers, by biosorption of an anionic azo dye, Congo red. Desalin Water Treat 54:245–254. https://doi.org/10.1080/19443994.2014.880154
Bouguettoucha A, Chebli D, Mekhalef T, Noui A, Amrane A (2014) The use of a forest waste biomass, cone of Pinus brutia for the removal of an anionic azo dye Congo red from aqueous medium. Desalin Water Treat 55:1956-1965. https://doi.org/10.1080/19443994.2014.928235
Reffas A, Bouguettoucha A, Chebli D, Amrane A (2016) Adsorption of ethyl violet dye in aqueous solution by forest wastes, wild carob. Desalin Water Treat 57:9859–9870. https://doi.org/10.1080/19443994.2015.1031707
Chebli D, Bouguettoucha A, Reffas A, Tiar C, Boutahala M, Gulyas H, Amrane A (2016) Removal of the anionic dye Biebrich scarlet from water by adsorption to calcined and non-calcined Mg–Al layered double hydroxides. Desalin Water Treat 57:22061–22073. https://doi.org/10.1080/19443994.2015.1128365
Gündüz F, Bayrak B (2017) Biosorption of malachite green from an aqueous solution using pomegranate peel: equilibrium modelling, kinetic and thermodynamic studies. J Mol Liq 243:790–798. https://doi.org/10.1016/j.molliq.2017.08.095
Kebaili M, Djellali S, Radjai M, Drouiche N, Lounici H (2018) Valorization of orange industry residues to form a natural coagulant and adsorbent. J Ind Eng Chem 64:292–299. https://doi.org/10.1016/j.jiec.2018.03.027
Hasdemir ZM, Şimşek S (2018) Removal of cationic dye in aquatic medium by using a new composite material. Cumhuriyet Sci J 39:181–191
Patel RK, Kumar S, Chawla AK, Mondal P, Neelam, Teychene B, Pandey JK (2019) Elimination of fluoride, arsenic, and nitrate from water through adsorption onto nano-adsorbent: a review. CNANO 15:557–575. https://doi.org/10.2174/1573413715666190101113651
Şenol ZM, Gürsoy N, Şimşek S, Özer A, Karakuş N (2020) Removal of food dyes from aqueous solution by chitosan-vermiculite beads. Int J Biol Macromol 148:635–646. https://doi.org/10.1016/j.ijbiomac.2020.01.166
Guediri A, Bouguettoucha A, Chebli D, Chafai N, Amrane A (2020) Molecular dynamic simulation and DFT computational studies on the adsorption performances of methylene blue in aqueous solutions by orange peel-modified phosphoric acid. J Mol Struct 1202:127290. https://doi.org/10.1016/j.molstruc.2019.127290
Guediri A, Bouguettoucha A, Chebli D, Amrane A (2020) The use of encapsulation as a proposed solution to avoid problems encountered with conventional materials in powder form: application in methylene blue removal from aqueous solutions. J Mol Liq 316:113841. https://doi.org/10.1016/j.molliq.2020.113841
Lin J, Wang L (2009) Comparison between linear and non-linear forms of pseudo-first-order and pseudo-second-order adsorption kinetic models for the removal of methylene blue by activated carbon. Front Environ Sci Eng China 3:320–324. https://doi.org/10.1007/s11783-009-0030-7
Simonin J-P (2016) On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem Eng J 300:254–263. https://doi.org/10.1016/j.cej.2016.04.079
Oladipo AA, Gazi M (2014) Enhanced removal of crystal violet by low cost alginate/acid activated bentonite composite beads: optimization and modelling using non-linear regression technique. J Water Process Eng 2:43–52. https://doi.org/10.1016/j.jwpe.2014.04.007
Tseng R-L, Wu P-H, Wu F-C, Juang R-S (2014) A convenient method to determine kinetic parameters of adsorption processes by nonlinear regression of pseudo-nth-order equation. Chem Eng J 237:153–161. https://doi.org/10.1016/j.cej.2013.10.013
Eastoe J, Dalton JS (2000) Dynamic surface tension and adsorption mechanisms of surfactants at the air–water interface. Adv Colloid Interf Sci 85:103–144. https://doi.org/10.1016/S0001-8686(99)00017-2
Sellaoui L, Bouzid M, Duclaux L, Reinert L, Knani S, Ben Lamine A (2016) Binary adsorption isotherms of two ionic liquids and ibuprofen on an activated carbon cloth: simulation and interpretations using statistical and COSMO-RS models. RSC Adv 6:67701–67714. https://doi.org/10.1039/C6RA03405E
Bouaziz N, Ben Manaa M, Aouaini F, Ben Lamine A (2019) Investigation of hydrogen adsorption on zeolites A, X and Y using statistical physics formalism. Mater Chem Phys 225:111–121. https://doi.org/10.1016/j.matchemphys.2018.12.024
Sellaoui L, Dotto GL, Lamine AB, Erto A (2017) Interpretation of single and competitive adsorption of cadmium and zinc on activated carbon using monolayer and exclusive extended monolayer models. Environ Sci Pollut Res 24:19902–19908. https://doi.org/10.1007/s11356-017-9562-8
Sellaoui L, Edi Soetaredjo F, Ismadji S, Cláudio Lima É, Dotto GL, Ben Lamine A, Erto A (2017) New insights into single-compound and binary adsorption of copper and lead ions on a treated sea mango shell: experimental and theoretical studies. Phys Chem Chem Phys 19:25927–25937. https://doi.org/10.1039/C7CP03770H
Lawal IA, Lawal MM, Akpotu SO, Azeez MA, Ndungu P, Moodley B (2018) Theoretical and experimental adsorption studies of sulfamethoxazole and ketoprofen on synthesized ionic liquids modified CNTs. Ecotoxicol Environ Saf 161:542–552. https://doi.org/10.1016/j.ecoenv.2018.06.019
Sellaoui L, Guedidi H, Knani S, Reinert L, Duclaux L, Ben Lamine A (2015) Application of statistical physics formalism to the modeling of adsorption isotherms of ibuprofen on activated carbon. Fluid Phase Equilib 387:103–110. https://doi.org/10.1016/j.fluid.2014.12.018
Silva LS, Lima LCB, Ferreira FJL et al (2015) Sorption of the anionic reactive red RB dye in cellulose: assessment of kinetic, thermodynamic, and equilibrium data. Open Chem 13. https://doi.org/10.1515/chem-2015-0079
El-Sikaily A, El Nemr A, Khaled A (2011) Copper sorption onto dried red alga Pterocladia capillacea and its activated carbon. Chem Eng J 168:707–714. https://doi.org/10.1016/j.cej.2011.01.064
Khelifa A (2001) Adsorption de CO2 par des zeolithes X echangees par des cations bivalents. Ann Chim Sci Mater 26:55–66. https://doi.org/10.1016/S0151-9107(01)80046-5
Şenol ZM, Gül ÜD, Gürkan R (2020) Bio-sorption of bisphenol a by the dried- and inactivated-lichen (Pseudoevernia furfuracea) biomass from aqueous solutions. J Environ Health Sci Eng. https://doi.org/10.1007/s40201-020-00508-6
Eren E (2008) Removal of copper ions by modified Unye clay, Turkey. J Hazard Mater 159:235–244. https://doi.org/10.1016/j.jhazmat.2008.02.035
Aouaini F, Souhail B, Khemiri N, Ben Yahia M, Almogait ES, AlHarbi FF, Almuqrin AH, Abdelmottaleb BL (2019) Study of the CO2 adsorption isotherms on El Hicha clay by statistical physics treatment: microscopic and macroscopic investigation. Sep Sci Technol 54:2577–2588. https://doi.org/10.1080/01496395.2018.1548487
Khattri SD, Singh MK (2009) Removal of malachite green from dye wastewater using neem sawdust by adsorption. J Hazard Mater 167:1089–1094. https://doi.org/10.1016/j.jhazmat.2009.01.101
Cherifi H, Fatiha B, Salah H (2013) Kinetic studies on the adsorption of methylene blue onto vegetal fiber activated carbons. Appl Surf Sci 282:52–59. https://doi.org/10.1016/j.apsusc.2013.05.031
Tan IAW, Hameed BH, Ahmad AL (2007) Equilibrium and kinetic studies on basic dye adsorption by oil palm fibre activated carbon. Chem Eng J 127:111–119. https://doi.org/10.1016/j.cej.2006.09.010
Harrache Z, Abbas M, Aksil T, Trari M (2019) Thermodynamic and kinetics studies on adsorption of indigo carmine from aqueous solution by activated carbon. Microchem J 144:180–189. https://doi.org/10.1016/j.microc.2018.09.004
Rahmi I, Mustafa I (2019) Methylene blue removal from water using H2SO4 crosslinked magnetic chitosan nanocomposite beads. Microchem J 144:397–402. https://doi.org/10.1016/j.microc.2018.09.032
Liu X, Cui B, Liu S, Ma Q (2019) Methylene blue removal by graphene oxide/alginate gel beads. Fibers Polym 20:1666–1672. https://doi.org/10.1007/s12221-019-9011-z
Bounaas M, Bouguettoucha A, Chebli D, Gatica JM, Vidal H (2020) Role of the wild carob as biosorbent and as precursor of a new high-surface-area activated carbon for the adsorption of methylene blue. Arab J Sci Eng. https://doi.org/10.1007/s13369-020-04739-5
Marrakchi F, Ahmed MJ, Khanday WA, Asif M, Hameed BH (2017) Mesoporous-activated carbon prepared from chitosan flakes via single-step sodium hydroxide activation for the adsorption of methylene blue. Int J Biol Macromol 98:233–239. https://doi.org/10.1016/j.ijbiomac.2017.01.119
Ravi PLM (2019) Enhanced adsorption capacity of designed bentonite and alginate beads for the effective removal of methylene blue. Appl Clay Sci 169:102–111. https://doi.org/10.1016/j.clay.2018.12.019
Milonjic S (2007) A consideration of the correct calculation of thermodynamic parameters of adsorption. J Serb Chem Soc 72:1363–1367. https://doi.org/10.2298/JSC0712363M
Acknowledgments
The authors would like to thank the MESRS and the DGRSDT (Ministère de l’Enseignement Supérieur et de la Recherche Scientifique et la Direction Générale de la Recherche Scientifique et du Développement Technologique - Algérie) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Djama, C., Chebli, D., Bouguettoucha, A. et al. Statistical physics modelling of azo dyes biosorption onto modified powder of Acorus calamus in batch reactor. Biomass Conv. Bioref. 13, 1013–1028 (2023). https://doi.org/10.1007/s13399-020-01190-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01190-2