Abstract
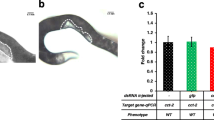
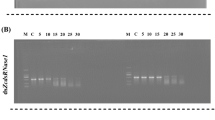
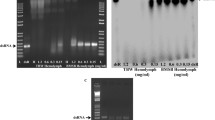
RNA interference (RNAi) is a common tool for analysis of gene function in both model and non-model insects, but it is becoming evident that RNAi efficiency varies considerably from species to species. We examined RNAi efficiency in larvae of the armyworm Mythimna separata (Walker) using multiple genes and tissues. First, we showed that five different target genes exhibited distinct tissue distribution patterns by quantitative determination of mRNA in total hemocytes, foregut, midgut, hindgut, Malpighian tubules and fat body: neuroglian mRNA was most abundant in fat body; inhibitor of apoptosis proteins mRNA was found to be ubiquitous; aquaporin 4 mRNA was most enriched in hindgut; cueball and prophenoloxidase 2 were mainly expressed in hemocytes. Second, we assessed sensitivity to gene silencing by double-strand RNA injection of these five genes in the six different tissues. We found that these genes generally showed refractoriness to double-strand RNA-mediated gene knockdown irrespective of the tissue tested. Finally, we demonstrated that appreciable gene knockdown was achieved at least in the adhering hemocyte fraction when larval isolated abdomen was prepared by ligation and subjected to dsRNA injection. Our study thus added detailed information on the refractoriness of larval tissues of a lepidopteran insect to gene silencing through RNAi and provided a new potential approach to improve RNAi efficiency.



Similar content being viewed by others
References
Bellés X (2010) Beyond Drosophila: RNAi in vivo and functional genomics in insects. Annu Rev Entomol 55:111–128
Borgnia M, Nielsen S, Engel A, Agre P (1999) Cellular and molecular biology of the aquaporin water channels. Annu Rev Biochem 68:425–458
Cerenius L, Söderhall K (2004) The prophenoloxidase-activating system in invertebrates. Immunol Rev 198:116–126
Cerenius L, Lee BL, Söderhall K (2008) The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol 29:263–271
Dzitoyeva S, Dimitrijevic N, Manev H (2001) Intra-abdominal injection of double-stranded RNA into anesthetized adult Drosophila triggers RNA interference in the central nervous system. Mol Psych 6:665–670
Dzitoyeva S, Dimitrijevic N, Manev H (2003) Identification of a novel Drosophila gene, beltless, using injectable embryonic and adult RNA interference (RNAi). Bmc Gen 4:14
Fire A, Xu SQ, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811
Garbutt JS, Bellés X, Richards EH, Reynolds SE (2013) Persistence of double-stranded RNA in insect hemolymph as a potential determiner of RNA interference success: evidence from Manduca sexta and Blattella germanica. J Insect Physiol 59:171–178
Godenschwege TA, Kristiansen LV, Uthaman SB, Hortsch M, Murphey RK (2006) A conserved role of Drosophila neuroglian and human L1-CAM in central-synapse formation. Curr Biol 16:12–23
Goto A, Blandin S, Royet J, Reichhart JM, Levashina EA (2003) Silencing of Toll pathway components by direct injection of double-stranded RNA into Drosophila adult flies. Nucl Acids Res 31:6619–6623
Hannon GJ (2002) RNA interference. Nature 418:244–251
Hirst J, Carmichael J (2011) A potential role for the clathrin adaptor GGA in Drosophila spermatogenesis. Bmc Cell Biol 12:22
Huang QH, Deveraux QL, Maeda S, Salvesen GS, Stennicke HR, Hammock BD, Reed JC (2000) Evolutionary conservation of apoptosis mechanisms: Lepidopteran and baculoviral inhibitor of apoptosis proteins are inhibitors of mammalian caspase-9. Proc Natl Acad Sci USA 97:1427–1432
Huvenne H, Smagghe G (2010) Mechanisms of dsRNA uptake in insects and potential of RNAi for pest control: a review. J Insect Physiol 56:227–235
Jose AM, Hunter CP (2007) Transport of sequence-specific RNA interference information between cells. Annu Rev Genet 41:305–330
Kocks C, Cho HJ, Nehme N, Ulvila J, Pearson AM, Meister M, Strom C, Conto SL, Hetru C, Stuart LM, Stehle T, Hoffmann JA, Reichhart J, Ferrandon D, Rämet M, Ezekowitz AB (2005) Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in Drosophila. Cell 123:335–346
Lavine MD, Strand MR (2002) Insect hemocyte and their role in immunity. Insect Biochem Mol Biol 32:1295–1309
Meister G, Tuschl T (2004) Mechanisms of gene silencing by double-stranded RNA. Nature 431:343–349
Miller SC, Brown SJ, Tomoyasu Y (2008) Larval RNAi in Drosophila? Dev Genes Evol 218:505–510
Mito T, Nakamura T, Bando T, Ohuchi H, Noji S (2011) The advent of RNA interference in entomology. Entomol Sci 14:1–8
Oda Y, Matsumoto H, Kurakake M, Ochiai M, Ohnishi A, Hayakawa Y (2010) Adaptor protein is essential for insect cytokine signaling in hemocytes. Proc Natl Acad Sci USA 107:15862–15867
Price DRG, Gatehouse JA (2008) RNAi-mediated crop protection against insects. Trends Biotech 26:393–400
Rämet M, Pearson A, Manfruelli P, Li X, Koziel H, Göbel V, Chung E, Krieger M, Alan R, Ezekowitz B (2001) Drosophila Scavenger Receptor CI is a pattern recognition receptor for bacteria. Immunity 15:1027–1038
Ribeiro C, Brehelin M (2006) Insect haemocytes: what type of cell is that? J Insect Physiol 52:417–429
Saleh MC, van Rij RP, Hekele A, Gillis A, Foley E, O’Farrell PH, Andino R (2006) The endocytic pathway mediates cell entry of dsRNA to induce RNAi silencing. Nat Cell Biol 8:793–802
Salvesen GS, Duckett CS (2002) IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol 3:401–410
Siomi H, Siomi MC (2009) On the road to reading the RNA-interference code. Nature 457:396–404
Suzuki M, Tanaka T (2007) Development of Meteorus pulchricornis and regulation of its noctuid host, Pseudaletia separata. J Insect Physiol 53:1072–1078
Suzuki M, Miura K, Tanaka T (2008) The virus-like particles of a braconid endoparasitoid wasp, Meteorus pulchricornis, inhibit hemocyte spreading in its noctuid host, Pseudaletia separata. J Insect Physiol 54:1015–1022
Suzuki M, Miura K, Tanaka T (2009) Effects of the virus-like particles of a braconid endoparasitoid, Meteorus pulchricornis, on hemocytes and hematopoietic organs of its noctuid host, Pseudaletia separata. Appl Entomol Zool 44:115–125
Terenius O, Papanicolaou A, Garbutt JS, Eleftherianos I, Huvenne H, Kanginakudru S, Albrechtsen M, An CJ, Aymeric JL, Barthel A, Bebas P, Bitra K, Bravo A, Chevalieri F, Collinge DP, Crava CM, de Maagd RA, Duvic B, Erlandson M, Faye I, Felfoldi G, Fujiwara H, Futahashi R, Gandhe AS, Gatehouse HS, Gatehouse LN, Giebultowicz JM, Gomez I, Grimmelikhuijzen CJP, Groot AT, Hauser F, Heckel DG, Hegedus DD, Hrycaj S, Huang LH, Hull JJ, Iatrou K, Iga M, Kanost MR, Kotwica J, Li CY, Li JH, Liu JS, Lundmark M, Matsumoto S, Meyering-Vos M, Millichap PJ, Monteiro A, Mrinal N, Niimi T, Nowara D, Ohnishi A, Oostra V, Ozaki K, Papakonstantinou M, Popadic A, Rajam MV, Saenko S, Simpson RM, Soberon M, Strand MR, Tomita S, Toprak U, Wang P, Wee CW, Whyard S, Zhang WQ, Nagaraju J, Ffrench-Constant RH, Herrero S, Gordon K, Swelters L, Smagghe G (2011) RNA interference in Lepidoptera: an overview of successful and unsuccessful studies and implications for experimental design. J Insect Physiol 57:231–245
Tomoyasu Y, Miller SC, Tomita S, Schoppmeier M, Grossmann D, Bucher G (2008) Exploring systemic RNA interference in insects: a genome-wide survey for RNAi genes in Tribolium. Gen Biol 9:R10
Ulvila J, Parikka M, Kleino A, Sormunen R, Ezekowitz RA, Kocks C, Ramet M (2006) Double-stranded RNA is internalized by scavenger receptor-mediated endocytosis in Drosophila S2 cells. J Biol Chem 281:14370–14375
Wynant N, Verlinden H, Breugelmans B, Simonet G, Vanden Broeck J (2012) Tissue-dependence and sensitivity of the systemic RNA interference response in the desert locust, Schistocerca gregaria. Insect Biochem Mol Biol 42:911–917
Yokoi K, Koyama H, Ito W, Minakuchi C, Tanaka T, Miura K (2012a) Involvement of NF-κB transcription factors in antimicrobial peptide gene induction in the red flour beetle, Tribolium castaneum. Dev Comp Immunol 38:342–351
Yokoi K, Koyama H, Minakuchi C, Tanaka T, Miura K (2012b) Antimicrobial peptide gene induction, involvement of Toll and IMD pathways and defense against bacteria in the red flour beetle, Tribolium castaneum. Results Immunol 2:72–82
Zhuang SF, Kelo L, Nardi JB, Kanost MR (2007) Neuroglian on hemocyte surfaces is involved in homophilic and heterophilic interactions of the innate immune system of Manduca sexta. Dev Comp Immunol 31:1159–1167
Acknowledgments
This work was supported in part by Grant-in-Aid 23658047 and 25450486, to KM from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yokoi, K., Kamezaki, M., Yoshida, T. et al. Sensitivity to RNA interference-mediated gene silencing in intact and ligated larvae of the armyworm, Mythimna separata (Lepidoptera: Noctuidae). Appl Entomol Zool 48, 431–439 (2013). https://doi.org/10.1007/s13355-013-0201-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-013-0201-7