Abstract
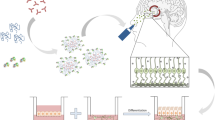
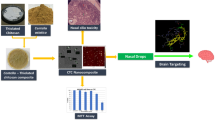
Nose-to-brain delivery is a promising approach to target drugs into the brain, avoiding the blood-brain barrier and other drawbacks related to systemic absorption, and enabling an effective and safer treatment of diseases such as glioblastoma (GBM). Innovative materials and technologies that improve residence time in the nasal cavity and modulate biological interactions represent a great advance in this field. Mucoadhesive nanoparticles (NPs) based on poly(lactic-co-glycolic acid) (PLGA) and oligomeric chitosan (OCS) were designed as a rational strategy and potential platform to co-deliver alpha-cyano-4-hydroxycinnamic acid (CHC) and the monoclonal antibody cetuximab (CTX) into the brain, by nasal administration. The influence of formulation and process variables (O/Aq volume ratio, Pluronic concentration, PLGA concentration, and sonication time) on the properties of CHC-loaded NPs (size, zeta potential, PDI and entrapment efficiency) was investigated by a two-level full factorial design (24). Round, stable nano-sized particles (213–875 nm) with high positive surface charge (+ 33.2 to + 58.9 mV) and entrapment efficiency (75.69 to 93.23%) were produced by the emulsification/evaporation technique. Optimal process conditions were rationally selected based on a set of critical NP attributes (258 nm, + 37 mV, and 88% EE) for further conjugation with CTX. The high cytotoxicity of CHC-loaded NPs and conjugated NPs was evidenced for different glioma cell lines (U251 and SW1088). A chicken chorioallantoic membrane assay highlighted the expressive antiangiogenic activity of CHC-loaded NPs, which was enhanced for conjugated NPs. The findings of this work demonstrated the potential of this nanostructured polymeric platform to become a novel therapeutic alternative for GBM treatment.

Graphical abstract











Similar content being viewed by others
References
Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392:432–46. https://doi.org/10.1016/S0140-6736(18)30990-5.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
Cavenee WK, Louis DN, Ohgaki H, Wiestler OD. WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. https://doi.org/10.1007/s00401-007-0243-4.
Akmal M, Hasnain N, Rehan A, Iqbal U, Hashmi S, Fatima K, et al. Glioblastome Multiforme: a bibliometric analysis. World Neurosurg. 2020;136:270–82. https://doi.org/10.1016/j.wneu.2020.01.027.
Wirsching H-G, Galanis E, Weller M. Chapter 23 - glioblastoma. In: Berger MS, Weller M, editors. Handbook of Clinical Neurology: Elsevier; 2016. p. 381–97.
Begley DJ. Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Therapeut. 2004;104:29–45. https://doi.org/10.1016/j.pharmthera.2004.08.001.
Alexander A, Agrawal M, Uddin A, Siddique S, Shehata AM, Shaker MA, et al. Recent expansions of novel strategies towards the drug targeting into the brain. Int J Nanomedicine. 2019;14:5895–909. https://doi.org/10.2147/ijn.s210876.
Portnow J, Badie B, Chen M, Liu A, Blanchard S, Synold TW. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin Cancer Res. 2009;15:7092–8. https://doi.org/10.1158/1078-0432.ccr-09-1349.
Quintana DS, Westlye LT. Low-dose intranasal oxytocin delivered with breath powered device modulates pupil diameter and amygdala activity: a randomized controlled pupillometry and fMRI study. Neuropsychopharcol. 2019;44:306–13. https://doi.org/10.1038/s41386-018-0241-3.
Chen TC, da Fonseca CO. Intranasal perillyl alcohol for glioma therapy: molecular mechanisms and clinical development. Int J Mol Sci. 2018;19:1–21. https://doi.org/10.3390/ijms19123905.
Djupesland PG, Messina JC, Mahmoud RA. The nasal approach to delivering treatment for brain diseases: an anatomic, physiologic, and delivery technology overview. Ther Deliv. 2014;5:709–33. https://doi.org/10.4155/tde.14.41.
Liu Q, Zhang Q. 10 - nanoparticle systems for nose-to-brain delivery. In: Gao H, Gao X, editors. Brain Targeted Drug Delivery System: Academic Press; 2019. p. 219–39. https://doi.org/10.1016/B978-0-12-814001-7.00010-X.
Carvalho FC, Campos ML, Peccinini RG, Gremiao MP. Nasal administration of liquid crystal precursor mucoadhesive vehicle as an alternative antiretroviral therapy. Eur J Pharm Biopharm. 2013;84:219–27. https://doi.org/10.1016/j.ejpb.2012.11.021.
Mittal D, Ali A, Md S, Baboota S, Sahni JK, Ali J. Insights into direct nose to brain delivery: current status and future perspective. Drug Deliv. 2014;21:75–86. https://doi.org/10.3109/10717544.2013.838713.
Rani V, Venkatesan J, Prabhu A. Nanotherapeutics in glioma management: advances and future perspectives. J Drug Deliv Sci Technol. 2020;57:1–37. https://doi.org/10.1016/j.jddst.2020.101626.
Nigam K, Kaur A, Tyagi A, Nematullah M, Khan F, Gabrani R, et al. Nose-to-brain delivery of lamotrigine-loaded PLGA nanoparticles. Drug Deliv Transl Re. 2019;9:879–90. https://doi.org/10.1007/s13346-019-00622-5.
Samaridou E, Alonso MJ. Nose-to-brain peptide delivery – the potential of nanotechnology. Bioorgan Med Chem. 2018;26:2888–905. https://doi.org/10.1016/j.bmc.2017.11.001.
Ferreira LMB, Alonso JD, Kiill CP, Ferreira NN, Buzzá HH, Martins de Godoi DR et al. Exploiting supramolecular interactions to produce bevacizumab-loaded nanoparticles for potential mucosal delivery Eur Polym J 2018; 103: 238–250. https://doi.org/10.1016/j.eurpolymj.2018.04.013.
Illum L. Nasal drug delivery--possibilities, problems and solutions. J Control Release. 2003;87:187–98. https://doi.org/10.1016/S0168-3659(02)00363-2.
Malhotra M, Tomaro-Duchesneau C, Saha S, Kahouli I, Prakash S. Development and characterization of chitosan-PEG-TAT nanoparticles for the intracellular delivery of siRNA. Int J Nanomedicine. 2013:2041–52. https://doi.org/10.2147/ijn.s43683.
Muanprasat C, Chatsudthipong V. Chitosan oligosaccharide: biological activities and potential therapeutic applications. Pharmacol Therapeut. 2017;170:80–97. https://doi.org/10.1016/j.pharmthera.2016.10.013.
Wesseling P, Capper D. WHO 2016 classification of gliomas. Neuropathol Appl Neurobiol. 2018;44:139–50. https://doi.org/10.1111/nan.12432.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. https://doi.org/10.1016/j.cell.2011.02.013.
Ferreira NN, Granja S, Boni FI, Ferreira LMB, Reis RM, Baltazar F, et al. A novel strategy for glioblastoma treatment combining alpha-cyano-4-hydroxycinnamic acid with cetuximab using nanotechnology-based delivery systems. Drug Deliv Trans Res. 2020;10:594–609. https://doi.org/10.1007/s13346-020-00713-8.
Abd Ellah NH, Abouelmagd SA. Surface functionalization of polymeric nanoparticles for tumor drug delivery: approaches and challenges. Expert Opin Drug Deliv. 2017;14:201–14. https://doi.org/10.1080/17425247.2016.1213238.
Noh J-K, Naeem M, Cao J, Lee EH, Kim M-S, Jung Y, et al. Herceptin-functionalized pure paclitaxel nanocrystals for enhanced delivery to HER2-postive breast cancer cells. Int J Pharm. 2016;513:543–53. https://doi.org/10.1016/j.ijpharm.2016.09.067.
Gomes MJ, Kennedy PJ, Martins S, Sarmento B. Delivery of siRNA silencing P-gp in peptide-functionalized nanoparticles causes efflux modulation at the blood–brain barrier. Nanomedicine : nanotechnology, biology and medicine. 2017;12:1385–99. https://doi.org/10.2217/nnm-2017-0023.
Kaluzova M, Bouras A, Machaidze R, Hadjipanayis CG. Targeted therapy of glioblastoma stem-like cells and tumor non-stem cells using cetuximab-conjugated iron-oxide nanoparticles. Oncotarget. 2015;6:8788–806. https://doi.org/10.18632/oncotarget.3554.
Win KY, Teng CP, Ye E, Low M, Han MY. Evaluation of polymeric nanoparticle formulations by effective imaging and quantitation of cellular uptake for controlled delivery of doxorubicin. Small. 2015;11:1197–204. https://doi.org/10.1002/smll.201402073.
Miranda-Goncalves V, Honavar M, Pinheiro C, Martinho O, Pires MM, Pinheiro C, et al. Monocarboxylate transporters (MCTs) in gliomas: expression and exploitation as therapeutic targets. Neuro-oncology. 2013;15:172–88. https://doi.org/10.1093/neuonc/nos298.
Mortensen JH, Jeppesen M, Pilgaard L, Agger R, Duroux M, Zachar V, et al. Targeted antiepidermal growth factor receptor (cetuximab) immunoliposomes enhance cellular uptake in vitro and exhibit increased accumulation in an intracranial model of glioblastoma multiforme. J Drug Deliv. 2013;2013:205–9. https://doi.org/10.1155/2013/209205.
Silva-Correia J, Miranda-Goncalves V, Salgado AJ, Sousa N, Oliveira JM, Reis RM, et al. Angiogenic potential of gellan-gum-based hydrogels for application in nucleus pulposus regeneration: in vivo study. Tissue Eng Pt A. 2012;18:1203–12. https://doi.org/10.1089/ten.TEA.2011.0632.
Ferreira NN, Ferreira LMB, Miranda-Goncalves V, Reis RM, Seraphim TV, Borges JC, et al. Alginate hydrogel improves anti-angiogenic bevacizumab activity in cancer therapy. Eur J Pharm Biopharm. 2017;119:271–82. https://doi.org/10.1016/j.ejpb.2017.06.028.
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–22. https://doi.org/10.1016/j.jconrel.2012.01.043.
Vasir JK, Labhasetwar V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv Drug Deliver Rev. 2007;59:718–28. https://doi.org/10.1016/j.addr.2007.06.003.
Shi Y, Xue J, Jia L, Du Q, Niu J, Zhang D. Surface-modified PLGA nanoparticles with chitosan for oral delivery of tolbutamide. Colloid Surface B. 2018;161:67–72. https://doi.org/10.1016/j.colsurfb.2017.10.037.
Chung Y-I, Kim JC, Kim YH, Tae G, Lee S-Y, Kim K, et al. The effect of surface functionalization of PLGA nanoparticles by heparin-or chitosan-conjugated Pluronic on tumor targeting. J Control Release. 2010;143:374–82. https://doi.org/10.1016/j.jconrel.2010.01.017.
Nava-Arzaluz MG, Piñón-Segundo E, Ganem-Rondero A, Lechuga-Ballesteros D. Single emulsion-solvent evaporation technique and modifications for the preparation of pharmaceutical polymeric nanoparticles. Recent Pat Drug Deliv Formul. 2012;6:209–23. https://doi.org/10.2174/187221112802652633.
Bonaccorso A, Musumeci T, Serapide MF, Pellitteri R, Uchegbu IF, Puglisi G. Nose to brain delivery in rats: effect of surface charge of rhodamine B labeled nanocarriers on brain subregion localization. Colloid Surfaces B. 2017;154:297–306. https://doi.org/10.1016/j.colsurfb.2017.03.035.
Motwani SK, Chopra S, Talegaonkar S, Kohli K, Ahmad FJ, Khar RK. Chitosan-sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: formulation, optimisation and in vitro characterisation. Eur J Pharm Biopharm. 2008;68:513–25. https://doi.org/10.1016/j.ejpb.2007.09.009.
Ahmad E, Feng Y, Qi J, Fan W, Ma Y, He H, et al. Evidence of nose-to-brain delivery of nanoemulsions: cargoes but not vehicles. Nanoscale. 2017;9:1174–83. https://doi.org/10.1039/c6nr07581a.
Gartziandia O, Herran E, Pedraz JL, Carro E, Igartua M, Hernandez RM. Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration. Colloid Surface B. 2015;134:304–13. https://doi.org/10.1016/j.colsurfb.2015.06.054.
Crucho CIC, Barros MT. Polymeric nanoparticles: a study on the preparation variables and characterization methods. Mater Sci Eng C. 2017;80:771–84. https://doi.org/10.1016/j.msec.2017.06.004.
Lee CY, Ooi IH. Preparation of temozolomide-loaded nanoparticles for glioblastoma multiforme targeting-ideal versus reality. Pharmaceutical. 2016;9:1–11. https://doi.org/10.3390/ph9030054.
Rassu G, Soddu E, Posadino AM, Pintus G, Sarmento B, Giunchedi P, et al. Nose-to-brain delivery of BACE1 siRNA loaded in solid lipid nanoparticles for Alzheimer’s therapy. Colloid Surfaces B. 2017;152:296–301. https://doi.org/10.1016/j.colsurfb.2017.01.031.
Deepika MS, Thangam R, Sheena TS, Vimala RTV, Sivasubramanian S, Jeganathan K, et al. Dual drug loaded PLGA nanospheres for synergistic efficacy in breast cancer therapy. Mat Sci Eng C-Mater. 2019;103:109716. https://doi.org/10.1016/j.msec.2019.05.001.
Arisoy S, Sayiner O, Comoglu T, Onal D, Atalay O, Pehlivanoglu B. In vitro and in vivo evaluation of levodopa-loaded nanoparticles for nose to brain delivery. Pharm Dev Technol. 2020;25(6):735–47. https://doi.org/10.1080/10837450.2020.1740257.
Jiang Y, Liu C, Zhai W, Zhuang N, Han T, Ding Z. The optimization design of lactoferrin loaded HupA nanoemulsion for targeted drug transport via intranasal route. Int J Nanomedicine. 2019;14:9217–34. https://doi.org/10.2147/IJN.S214657.
Tzeyung AS, Md S, Bhattamisra SK, Madheswaran T, Alhakamy NA, Aldawsari HM, et al. Fabrication, optimization, and evaluation of rotigotine-loaded chitosan nanoparticles for nose-to-brain delivery. Pharmaceutic. 2019;11:26. https://doi.org/10.3390/pharmaceutics11010026.
Kumar M, Pandey RS, Patra KC, Jain SK, Soni ML, Dangi JS, et al. Evaluation of neuropeptide loaded trimethyl chitosan nanoparticles for nose to brain delivery. Int J Biol Macromol. 2013;61:189–95. https://doi.org/10.1016/j.ijbiomac.2013.06.041.
Patel VR, Agrawal YK. Nanosuspension: an approach to enhance solubility of drugs. J Adv Pharm Technol Res. 2011;2:81–7. https://doi.org/10.4103/2231-4040.82950.
Bhattacharjee S. DLS and zeta potential–what they are and what they are not? J Control Release. 2016;235:337–51. https://doi.org/10.1016/j.jconrel.2016.06.017.
Schatz C, Domard A, Viton C, Pichot C, Delair T. Versatile and efficient formation of colloids of biopolymer-based polyelectrolyte complexes. Biomacromol. 2004;5:1882–92. https://doi.org/10.1021/bm049786+.
Nagavarma B, Yadav HK, Ayaz A, Vasudha L, Shivakumar H. Different techniques for preparation of polymeric nanoparticles-a review. Asian J Pharm Clin Res. 2012;5:16–23.
Madkour M, Bumajdad A, Al-Sagheer F. To what extent do polymeric stabilizers affect nanoparticles characteristics? Adv Colloid Interfac. 2019;270:38–53. https://doi.org/10.1016/j.cis.2019.05.004.
Kharisov BI, Dias HR, Kharissova OV, Vázquez A, Pena Y, Gomez I. Solubilization, dispersion and stabilization of magnetic nanoparticles in water and non-aqueous solvents: recent trends. RSC Adv. 2014;4(85):45354–81. https://doi.org/10.1039/C4RA06902A.
Vilaça N, Amorim R, Martinho O, Reis RM, Baltazar F, Fonseca AM, et al. Encapsulation of α-cyano-4-hydroxycinnamic acid into a NaY zeolite. J Mat Sci. 2011;46:7511–6. https://doi.org/10.1007/s10853-011-5722-2.
Binder ZA, Thorne AH, Bakas S, Wileyto EP, Bilello M, Akbari H, et al. Epidermal growth factor receptor extracellular domain mutations in glioblastoma present opportunities for clinical imaging and therapeutic development. Cancer cell. 2018;34:163–77.e7. https://doi.org/10.1016/j.ccell.2018.06.006.
Hicks MJ, Chiuchiolo MJ, Ballon D, Dyke JP, Aronowitz E, Funato K, et al. Anti-epidermal growth factor receptor gene therapy for glioblastoma. PLoS One. 2016;11:e0162978. https://doi.org/10.1371/journal.pone.0162978.
Costa BM, Viana-Pereira M, Fernandes R, Costa S, Linhares P, Vaz R, et al. Impact of EGFR genetic variants on glioma risk and patient outcome. Cancer Epidemiol Biomark Prev. 2011;20:2610–7. https://doi.org/10.1158/1055-9965.epi-11-0340.
Viana-Pereira M, Lopes JM, Little S, Milanezi F, Basto D, Pardal F, et al. Analysis of EGFR overexpression, EGFR gene amplification and the EGFRvIII mutation in Portuguese high-grade gliomas. Anticancer Res. 2008;28:913–20.
Fukai J, Nishio K, Itakura T, Koizumi F. Antitumor activity of cetuximab against malignant glioma cells overexpressing EGFR deletion mutant variant III. Cancer Sci. 2008;99(10):2062–9. https://doi.org/10.1111/j.1349-7006.2008.00945.x.
Marques A, Costa P, Velho S, Amaral M. Functionalizing nanoparticles with cancer-targeting antibodies: a comparison of strategies. J Control Release. 2020;320:180–200. https://doi.org/10.1016/j.jconrel.2020.01.035.
Field LD, Delehanty JB, Chen Y, Medintz IL. Peptides for specifically targeting nanoparticles to cellular organelles: quo vadis? Account Chem Res. 2015;48(5):1380–90. https://doi.org/10.1021/ar500449v.
Tseng S-H, Chou M-Y, Chu IM. Cetuximab-conjugated iron oxide nanoparticles for cancer imaging and therapy. Int J Nanomedicine. 2015;10:3663–85. https://doi.org/10.2147/IJN.S80134.
Orellana EA, Kasinski AL, Sulforhodamine B. (SRB) assay in cell culture to investigate cell proliferation. Bio-protocol. 2016;6:e1984. https://doi.org/10.21769/BioProtoc.1984.
Turabee MH, Jeong TH, Ramalingam P, Kang JH, Ko YT. N, N, N-trimethyl chitosan embedded in situ Pluronic F127 hydrogel for the treatment of brain tumor. Carbohydr Polym. 2019;203:302–9. https://doi.org/10.1016/j.carbpol.2018.09.065.
Meng X, Liu J, Yu X, Li J, Lu X, Shen T. Pluronic F127 and D-α-tocopheryl polyethylene glycol succinate (TPGS) mixed micelles for targeting drug delivery across the blood brain barrier. Sci Rep. 2017;7(1):1–12. https://doi.org/10.1038/s41598-017-03123-y.
Mauriz JL, González-Gallego J. Antiangiogenic drugs: current knowledge and new approaches to cancer therapy. J Pharm Sci. 2008;97:4129–54. https://doi.org/10.1002/jps.21286.
Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, et al. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. T Am J Pathol. 1997;151:1523–30.
Vincenzi B, Zoccoli A, Pantano F, Venditti O, Galluzzo S. Cetuximab: from bench to bedside. Curr Cancer Drug Targets. 2010;10:80–95. https://doi.org/10.2174/156800910790980241.
Peek M, Norman T, Morgan C, Markham R, Fraser I. The chick chorioallantoic membrane assay: an improved technique for the study of angiogenic activity. Exp Pathol. 1988;34:35–40. https://doi.org/10.1016/S0232-1513(88)80020-3.
Price J, Barry M, Andrews I. The use of the chick chorioallantoic membrane to predict eye irritants. Food Chem Toxicol. 1986;24:503–5. https://doi.org/10.1016/0278-6915(86)90101-8.
Vargas A, Zeisser-Labouèbe M, Lange N, Gurny R, Delie F. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv Drug Deliv Rev. 2007;59:1162–76. https://doi.org/10.1016/j.addr.2007.04.019.
Xiong C, Wu H, Wei P, Pan M, Tuo Y, Kusakabe I, et al. Potent angiogenic inhibition effects of deacetylated chitohexaose separated from chitooligosaccharides and its mechanism of action in vitro. Carbohydr Res. 2009;344:1975–83. https://doi.org/10.1016/j.carres.2009.06.036.
Vincenzi B, Schiavon G, Silletta M, Santini D, Tonini G. The biological properties of cetuximab. Critical Rev Onc Hemat. 2008;68:93–106. https://doi.org/10.1016/j.critrevonc.2008.07.006.
Acknowledgments
The authors would like to thank the National Institute of Science and Technology in Pharmaceutical Nanotechnology: a transdisciplinary approach INCT-NANOFARMA, which is supported by the “Fundação de Amparo e Pesquisa do Estado de São Paulo” (FAPESP, Brazil), Grant #2014/50928-2, and by “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq, Brazil), Grant # 465687/2014-8.
Funding
This work was financially supported by FAPESP Grant # 2017/16324-0. NNF received fellowships from FAPESP, ref. #2016/09671-3 and #2018/ 04546-1. This work has been funded through the Foundation for Science and Technology (FCT) - project UIDB/50026/2020 and UIDP/50026/2020; and by the projects NORTE-01-0145-FEDER-000013 and NORTE-01-0145-FEDER-000023, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). SG received a fellowship from FCT, ref. SFRH/BPD/117858/2016.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, and data acquisition: N.N.F., S.G., L.M.B.F., F.G.P., F.I.B. B.S.F.C; resources: M.P.D.G., F.B., R.M.R.; data curation: all authors; writing of original draft: N.N.F., B.S.F.C., F.G.P., F.I.B. L.M.B.F.; writing review and editing: all authors; supervision: M.P.D.G., B.S.F.C., F.B., R.M.R. project administration and funding acquisition: M.P.D.G., F.B.; data analysis and scientific discussion: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All experiments shown here comply with the current laws of the country in which they were performed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ferreira, N.N., Granja, S., Boni, F.I. et al. Modulating chitosan-PLGA nanoparticle properties to design a co-delivery platform for glioblastoma therapy intended for nose-to-brain route. Drug Deliv. and Transl. Res. 10, 1729–1747 (2020). https://doi.org/10.1007/s13346-020-00824-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00824-2