Abstract
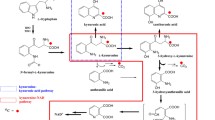
Tryptophan metabolites in plasma samples from 20 male subjects with type 2 diabetes mellitus (T2DM) and 20 nondiabetic reference males were analyzed by ultra high performance liquid chromatography. Tryptophan levels in the diabetic subjects were significantly lower than those in nondiabetic subjects. The concentrations of 5-hydroxytryptophan, 5-hydroxyindoleacetic acid, kynurenic acid, 3-hydroxykynurenine, 3-hydroxyanthranilic acid, and xanthurenic acid were found to be higher in the diabetic patients. When the diabetic patients were divided into higher- and lower-tryptophan groups, the concentrations of 5-hydroxytryptophan, indole-3-acetic acid, kynurenine, 5-hydroxykynurenine, and kynurenic acid were found to be higher in the diabetic patients with higher tryptophan levels. However, diabetic patients with lower plasma tryptophan levels had higher levels of 5-hydroxyindoleacetic acid than the patients with higher tryptophan levels. These results suggest that tryptophan was metabolized more in T2DM patients than in nondiabetic subjects. In the kynurenine pathway, the degradation of tryptophan seems to be accelerated in patients with higher plasma levels of tryptophan than in patients with lower levels of tryptophan. In the serotonin pathway, when the level of tryptophan is low, the conversion of serotonin to 5-hydroxyindoleacetic acid appears to be accelerated. In conclusion, our results suggest that T2DM patients may be exposed to stress constantly.

Similar content being viewed by others
References
Eussen SJ, Ueland PM, Vollset SE, Nygård O, Midttun Ø, Sulo G, Ulvik A, Meyer K, Pedersen ER, Tell GS. Kynurenines as predictors of acute coronary events in the Hordaland Health Study. Int J Cardiol. 2015;189:18–24.
Pedersen ER, Tuseth N, Eussen SJ, Ueland PM, Strand E, Svingen GF, Midttun Ø, Meyer K, Mellgren G, Ulvik A, Nordrehaug JE, Nilsen DW, Nygård O. Associations of plasma kynurenines with risk of acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol. 2015;35:455–62.
Adell A, Garcia-Marquez C, Armario A, Gelpi E. Chronic stress increases serotonin and noradrenaline in rat brain and sensitizes their responses to a further acute stress. J Neurochem. 1988;5016:78–81.
Price LH, Charney DS, Delgado PL, Goodman WK, Krystal JH, Woods SW, Heninger GR. Clinical studies of 5-HT function using i.v. l-tryptophan. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:459–72.
Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–79.
Malyszko J, Urano T, Yan D, Serizawa K, Kozima Y, Takada Y, Takada A. Foot shock-induced changes in blood and brain serotonin and related substances in rats. Jpn J Physiol. 1994;44:35–47.
Malyszko J, Urano T, Takada Y, Takada A. Amino acids, serotonin, and 5-hydroxyindoleacetic acid following foot shock in rats. Brain Res Bull. 1995;36:137–40.
Pawlak D, Takada Y, Urano T, Takada A. Serotonergic and kynurenic pathways in rats exposed to foot shock. Brain Res Bull. 2000;53:197–206.
Okuno E, Nishikawa T, Nakamura M. Kynurenine aminotransferases in the rat. Localization and characterization. Adv Exp Med Biol. 1996;398:455–64.
Niinisalo P, Oksala N, Levula M, Pelto-Huikko M, Järvinen O, Salenius JP, Kytömäki L, Soini JT, Kähönen M, Laaksonen R, Hurme M, Lehtimäki T. Activation of indoleamine 2,3-dioxygenase-induced tryptophan degradation in advanced atherosclerotic plaques: Tampere Vascular Study. Ann Med. 2010;42:55–63.
Oxenkrug G. Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol Neurobiol. 2013;48:294–301.
Moffett JR, Price RA, Anderson SM, Sipos ML, Moran AV, Tortella FC, Dave JR. DNA fragmentation in leukocytes following subacute low-dose nerve agent exposure. Cell Mol Life Sci. 2003;60:2266–71.
Nakagami Y, Saito H, Katsuki H. 3-Hydroxykynurenine toxicity on the rat striatum in vivo. Jpn J Pharmacol. 1996;2:183–6.
Okuda S, Nishiyama N, Saito H, Katsuki H. Basic fibroblast growth factor and brain-derived neurotrophic factor promote survival and neuronal circuit formation in organotypic hippocampal culture. J Neurochem. 1998;70:299–307.
Schwarcz R, Du F. Quinolinic acid and kynurenic acid in the mammalian brain. Adv Exp Med Biol. 1991;294:185–99.
Fuertig R, Azzinnari D, Bergamini G, Cathomas F, Sigrist H, Seifritz E, Vavassori S, Luippold A, Hengerer B, Ceci A, Pryce CR. Mouse chronic social stress increases blood and brain kynurenine pathway activity and fear behaviour: both effects are reversed by inhibition of indoleamine 2,3-dioxygenase. Brain Behav Immun. 2016;54:59–72.
Vecchiarelli HA, Gandhi CP, Hill MN. Acute psychological stress modulates the expression of enzymes involved in the kynurenine pathway throughout corticolimbic circuits in adult male rats. Neural Plast. 2016;2016:7215684. doi:10.1155/2016/7215684 (Epub 2015 Dec 27).
Takao T, Ogawa M, Ishii Y, Shimizu F, Takada A. Different glycemic resposes to sucrose and glucose in old and young male adults. J Nutr Food Sci. 2016;6:460. doi:10.4172/2155-9600.1000460.
Acknowledgments
The experiments were designed and performed by all of the authors. AT wrote the manuscript. Statistical analyses were done by TT. All of the authors read and approved the final manuscript, and take responsibility for the final content.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial support
This study was supported by grants from the Ito Memorial Foundation and the NPO International Projects on Food and Health.
Conflict of interest
M. Ogawa, T. Takao, Y. Ishii, F. Shimizu, J. Masuda, and T. Takada declare that they have no conflict of interest.
Ethics
This work was approved by the ethical committees of Showa Women’s University and the NPO International Projects on Food and Health, and was carried out in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki) for experiments.
About this article
Cite this article
Matsuoka, K., Kato, K., Takao, T. et al. Concentrations of various tryptophan metabolites are higher in patients with diabetes mellitus than in healthy aged male adults. Diabetol Int 8, 69–75 (2017). https://doi.org/10.1007/s13340-016-0282-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-016-0282-y