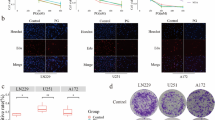
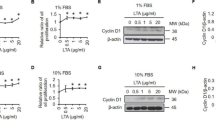
Abstract
As the first member of glycylcycline bacteriostatic agents, tigecycline is approved as a novel expanded-spectrum antibiotic, which is clinically available. However, accumulating evidence indicated that tigecycline was provided with the potential application in cancer therapy. In this paper, tigecycline was shown to exert an anti-proliferative effect on neuroblastoma cell lines. Furthermore, it was found that tigecycline induced G1-phase cell cycle arrest instead of apoptosis by means of Akt pathway inhibition. In neuroblastoma cell lines, the Akt activator insulin-like growth factor-1 (hereafter referred to as IGF-1) reversed tigecycline-induced cell cycle arrest. Besides, tigecycline inhibited colony formation and suppressed neuroblastoma cells xenograft formation and growth. After tigecycline treatment in vivo, the Akt pathway inhibition was confirmed as well. Collectively, our data provided strong evidences that tigecycline inhibited neuroblastoma cells growth and proliferation through the Akt pathway inhibition in vitro and in vivo. In addition, these results were supported by previous studies concerning the application of tigecycline in human tumors treatment, suggesting that tigecycline might act as a potential candidate agent for neuroblastoma treatment.





Similar content being viewed by others
References
Weinstein JL, Katzenstein HM, Cohn SL. Advances in the diagnosis and treatment of neuroblastoma. Oncologist. 2003;8:278–92.
Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–16.
Schleiermacher G, Janoueix-Lerosey I, Delattre O. Recent insights into the biology of neuroblastoma. Int J Cancer. 2014;135:2249–61.
Maris JM. Medical progress: recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–11.
Castel V, Grau E, Noguera R, Martinez F. Molecular biology of neuroblastoma. Clin Transl Oncol. 2007;9:478–83.
Livermore DM. Tigecycline: what is it, and where should it be used? J Antimicrob Chemother. 2005;56:611–4.
Nakazato A, Ohta K, Sekiguchi Y, Okuyama S, Chaki S, Kawashima Y, et al. Design, synthesis, structure-activity relationships, and biological characterization of novel arylalkoxyphenylalkylamine σ ligands as potential antipsychotic drugs. J Med Chem. 1999;42:1076–87.
Petersen PJ, Jacobus N, Weiss W, Sum P, Testa R. In vitro and in vivo antibacterial activities of a novel glycylcycline, the 9-t-butylglycylamido derivative of minocycline (gar-936). Antimicrob Agents Chemother. 1999;43:738–44.
Bergeron J, Ammirati M, Danley D, James L, Norcia M, Retsema J, et al. Glycylcyclines bind to the high-affinity tetracycline ribosomal binding site and evade tet(m)- and tet(o)-mediated ribosomal protection. Antimicrob Agents Chemother. 1996;40:2226–8.
Lamb R, Ozsvari B, Lisanti CL, Tanowitz HB, Howell A, Martinez-Outschoorn UE, et al. Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: treating cancer like an infectious disease. Oncotarget. 2015;6:4569–84.
Chen Z, Wang Y, Liu W, Zhao G, Lee S, Balogh A, et al. Doxycycline inducible Kruppel-like factor 4 lentiviral vector mediates mesenchymal to epithelial transition in ovarian cancer cells. PLoS One. 2014;9:e105331.
Liu W-T, Lin C-H, Hsiao M, Gean P-W. Minocycline inhibits the growth of glioma by inducing autophagy. Autophagy. 2011;7:166–75.
Tang C, Yang L, Jiang X, Xu C, Wang M, Wang Q, et al. Antibiotic drug tigecycline inhibited cell proliferation and induced autophagy in gastric cancer cells. Biochem Biophys Res Commun. 2014;446:105–12.
Li H, Jiao S, Li X, Banu H, Hamal S, Wang X. Therapeutic effects of antibiotic drug tigecycline against cervical squamous cell carcinoma by inhibiting wnt/beta-catenin signaling. Biochem Biophys Res Commun. 2015;467:14–20.
Bucaneve G, Micozzi A, Picardi M, Ballanti S, Cascavilla N, Salutari P, et al. Results of a multicenter, controlled, randomized clinical trial evaluating the combination of piperacillin/tazobactam and tigecycline in high-risk hematologic patients with cancer with febrile neutropenia. J Clin Oncol : Off J Am Soc Clin Oncol. 2014;32:1463–71.
Opel D, Poremba C, Simon T, Debatin K-M, Fulda S. Activation of Akt predicts poor outcome in neuroblastoma. Cancer Res. 2007;67:735–45.
West KA, Sianna Castillo S, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat. 2002;5:234–48.
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–68.
Greer EL, Brunet A. Foxo transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–25.
Guo L, Xie B, Mao Z. Autophagy in premature senescent cells is activated via AMPK pathway. Int J Mol Sci. 2012;13:3563–82.
de Mattos SF, Essafi A, Soeiro I, Pietersen AM, Birkenkamp KU, Edwards CS, et al. Foxo3a and bcr-abl regulate cyclin d2 transcription through a stat5/bcl6-dependent mechanism. Mol Cell Biol. 2004;24:10058–71.
Essafi A, Fernandez de Mattos S, Hassen YA, Soeiro I, Mufti GJ, Thomas NS, et al. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene. 2005;24:2317–29.
Besson A, Dowdy SF, Roberts JM. Cdk inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14:159–69.
Kurihara S, Hakuno F. Takahashi S-i: insulin-like growth factor-i-dependent signal transduction pathways leading to the induction of cell growth and differentiation of human neuroblastoma cell line sh-sy5y: the roles of map kinase pathway and pi 3-kinase pathway. Endocr J. 2000;47:739–51.
Yan X, Ke XX, Zhao H, Huang M, Hu R, Cui H. Triptolide inhibits cell proliferation and tumorigenicity of human neuroblastoma cells. Mol Med Rep. 2015;11:791–6.
Noskin GA. Tigecycline: a new glycylcycline for treatment of serious infections. Clin Infect Dis. 2005;41:S303–14.
Giamarellou H, Poulakou G. Pharmacokinetic and pharmacodynamic evaluation of tigecycline. Expert Opin Drug Metab Toxicol. 2011;7:1459–70.
Jitkova Y, Gronda M, Hurren R, Wang X, Goard CA, Jhas B, et al. A novel formulation of tigecycline has enhanced stability and sustained antibacterial and antileukemic activity. PLoS One. 2014;9:e95281.
Garcia Z, Kumar A, Marques M, Cortes I, Carrera AC. Phosphoinositide 3-kinase controls early and late events in mammalian cell division. EMBO J. 2006;25:655–61.
Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19.
Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603.
Fekete M, Santiskulvong C, Eng C, Dorigo O. Effect of PI3K/Akt pathway inhibition-mediated g1 arrest on chemosensitization in ovarian cancer cells. Anticancer Res. 2012;32:445–52.
Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol : CB. 1997;7:261–9.
Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346:561–76.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101.
Vincent EE, Elder DJ, Thomas EC, Phillips L, Morgan C, Pawade J, et al. Akt phosphorylation on Thr308 but not on Ser473 correlates with Akt protein kinase activity in human non-small cell lung cancer. Br J Cancer. 2011;104:1755–61.
Zheng WH, Quirion R. Insulin-like growth factor-1 (igf-1) induces the activation/phosphorylation of Akt kinase and camp response element-binding protein (CREB) by activating different signaling pathways in pc12 cells. BMC Neurosci. 2006;7:51.
Acknowledgments
This study was supported by the National Basic Research Program of China (No. 2012cb114603), National Nature Science Foundation of China (81201551), and the Fundamental Research Funds for the central universities (swu111014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Xiaoxia Zhong and Erhu Zhao contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
IGF-1 rescued cell cycle arrest induced by tigecycline treatment. Cell cycle was analyzed by FACS assay. BE2C and SK-N-AS cells were pretreated with 10 μM tigecycline for 48 h. After this, the cells were treated with 10nM IGF-1 for the indicated time. BE2C and SK-N-AS cells were harvested, fixed with 75 % ethanol, stained with PI, and analyzed by FACS assay. DMSO was used as a control.
Rights and permissions
About this article
Cite this article
Zhong, X., Zhao, E., Tang, C. et al. Antibiotic drug tigecycline reduces neuroblastoma cells proliferation by inhibiting Akt activation in vitro and in vivo. Tumor Biol. 37, 7615–7623 (2016). https://doi.org/10.1007/s13277-015-4613-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4613-6