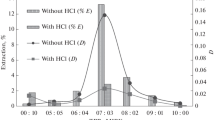
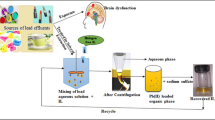
Abstract
In the present study, for the first time, the effect of sodium acetate, sodium citrate, and the addition of sodium oxalate was investigated in the D2EHPA system for the extraction of nickel and copper. In this study, the optimum condition including pH, the concentrations of additives, and time was found according to the separation factor and the extraction efficiencies. In this regard, the best extractant/extractants or their mixtures were found. Furthermore, the slope analysis was applied to understand the mechanism of extraction. According to the results, the system containing an optimum concentration of sodium oxalate and a mixture of sodium acetate and sodium oxalate could be used to separate copper from nickel and nickel from copper, respectively.










Similar content being viewed by others
References
Gharabaghi M, Irannajad M, and Azadmehr A R, Physicochem Probl Miner Process49 (2013) 233.
Sole K C, and Hiskey J B, Hydrometallurgy37 (1995) 129.
Lan Z, Hu Y, Liu J, and Wang J, J Cent South Univ Technol12 (2005) 45.
Agarwal S, Ferreira A E, Santos S M, Reis M T A, Ismael M R C, Correia M J N, and Carvalho J M, Int J Miner Process97 (2010) 85.
Mishra S, and Devi N, Hydrometallurgy107 (2011) 29.
Sinha M K, Sahu S, Meshram P, Pandey B, and Kumar V, Int J Metall Eng1 (2012) 28.
Agrawal A, Manoj M, Kumari S, Bagchi D, Kumar V, and Pandey B, Miner Eng21 (2008) 1126.
Padhan E, Sarangi K, and Subbaiah T, Int J Miner Process126 (2014) 55.
Reddy B R, and Park K H, Sep Sci Technol42 (2007) 2067.
Sridhar V, Verma J, and Shenoy N S, Miner Eng23 (2010) 454.
Sridhar V, and Verma J, J Chem8 (2011) S434.
Kumar R, Shah D J, and Tiwari K K, J Environ Prot4 (2013) 315.
Cheng C Y, Hydrometallurgy84 (2006) 109.
Haghshenas Fatmehsari D, Darvishi D, Etemadi S, Eivazi Hollagh A R, Keshavarz Alamdari E, and Salardini A A, Hydrometallurgy98 (2009) 143.
Molinari R, Poerio T, and Argurio P, J Membr Sci280 (2006) 470.
Huynh H T, and Tanaka M, Ind Eng Chem Res42 (2003) 4050.
Begum N, Bari F, Jamaludin S B, and Hussin K, Int J Phys Sci7 (2012) 2905.
Nikam G, and Mohite B, Res J Chem Sci2231 (2012) 606X.
Guezzen B, and Didi M A, Int. J. Chem.4 (2012) 32.
Nadimi H, Haghshenas Fatmehsari D, and Firoozi S, Metall Mater Trans B48 (2017) 2751.
Nadimi H, Amirjani A, Fatmehsari D H, Firoozi S, and Azadmehr A, Miner Eng69 (2014) 177.
Van de Voorde I, Pinoy L, Courtijn E, and Verpoort F, Solvent Extr. Ion Exch.24 (2006) 893.
Ren Z, Zhang W, Meng H, Liu Y, and Dai Y, J Chem Eng Data52 (2007) 438.
Van de Voorde I, Pinoy L, Courtijn E, and Verpoort F, Hydrometallurgy78 (2005) 92.
Correa M M J, Silvas F P C, Aliprandini P, Moraes V T d, Dreisinger D, and Espinosa D C R, Braz J Chem Eng35 (2018) 919.
Aksu S, J Electrochem Soc152 (2005) G938.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Irannajad, M., Kamran Haghighi, H. & Nasirpour, Z. New Solvent Extraction Process of Nickel and Copper by D2EHPA in the Presence of Carboxylates. Trans Indian Inst Met 73, 1053–1063 (2020). https://doi.org/10.1007/s12666-020-01940-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-020-01940-w