Abstract
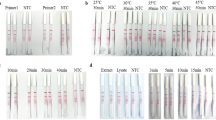
Human adenoviruses (hAdVs) of subgroup F (enteric serotypes 40 and 41) display characteristic gut tropism, in vivo, fastidious growth characteristics in cell culture, and are estimated to be associated with 5–20 % worldwide of acute gastroenteritis cases among infants and young children. Adequate hAdV gastroenteritis case management requires laboratory-based diagnosis. The present study aimed to the development and evaluation of a simple and cost-effective, one-step, single-tube adenovirus type 40/41 specific loop-mediated isothermal amplification (LAMP) assay for the detection of hAdV40/41 DNA in environmental and/or clinical samples, since no LAMP assay has previously been reported for the detection of these virus types. The assay targeted the hexon gene and had the advantages of being rapid, simple, specific, and sensitive. Results could be obtained within 60 min, under isothermal conditions at 69 °C. The detection limits for hAdV genomes were between 50 and 100 copies/reaction for hAdV40 and hAdV41, and no cross-reactions with other selected viruses, were found. The assay was evaluated with clinical as well as environmental samples. The developed assay is expected to provide a potential molecular tool in obtaining greater knowledge of the hAdV40/41 importance in the epidemiology and clinical manifestations of gastroenteritis.






Similar content being viewed by others
References
Albinana-Gimenez, N., Clemente-Casares, P., Calgua, B., Huguet, J. M., Courtois, S., & Girones, R. (2009a). Comparison of methods for concentrating human adenoviruses, polyomavirus JC and noroviruses in source waters and drinking water using quantitative PCR. Journal of Virological Methods, 158(1–2), 104–109.
Albinana-Gimenez, N., Miagostovich, M. P., Calgua, B., Huguet, J. M., Matia, L., & Girones, R. (2009b). Analysis of adenoviruses and polyoma viruses quantified by qPCR as indicators of water quality in source and drinking-water treatment plants. Water Research, 43(7), 2011–2019.
Allard, A., Albinsson, B., & Wadell, G. (2001). Rapid typing of human adenoviruses by a general PCR combined with restriction endonuclease analysis. Journal of Clinical Microbiology, 39(2), 498–505.
Bányai, K., Kisfali, P., Bogdán, A., Martella, V., Melegh, B., Erdman, D., & Szucs, G. (2009). Adenovirus gastroenteritis in Hungary, 2003-2006. European Journal of Clinical Microbiology and Infectious Diseases, 28, 997–999.
Bofill-Mas, S., Albinana-Gimenez, N., Clemente-Casares, P., Hundesa, A., Rodriguez-Manzano, J., Allard, A., et al. (2006). Quantification and stability of human adenoviruses and polyomavirus JCPyV in wastewater matrices. Applied and Environmental Microbiology, 72(12), 7894–7896.
Buckwalter, S. P., Teo, R., Espy, M. J., Sloan, L. M., Smith, T. F., & Pritt, B. S. (2012). Real-time qualitative PCR for 57 human adenovirus types from multiple specimen sources. Journal of Clinical Microbiology, 50(3), 766–771.
Calgua, B., Mengewein, A., Grunert, A., Bofill-Mas, S., Clemente-Casares, P., Hundesa, A., et al. (2008). Development and application of a one-step low cost procedure to concentrate viruses from seawater samples. Journal of Virological Methods, 153(2), 79–83.
Calgua, B., Rodriguez-Manzano, J., Hundesa, A., Suñen, E., Calvo, M., Bofill-Mas, S., & Girones, R. (2013). New methods for the concentration of viruses from urban sewage using quantitative PCR. Journal of Virological Methods, 187(2), 215–221.
Chapron, C. D., Ballester, N. A., Fontaine, J. H., Frades, C. N., & Margolin, A. B. (2000). Detection of astroviruses, enteroviruses, and adenovirus types 40 and 41 in surface waters collected and evaluated by the information collection rule and an integrated cell culture-nested PCR procedure. Applied and Environmental Microbiology, 66, 2520–2525.
De Jong, J. C., Wigand, R., Kidd, A. H., Wadell, G., Kapsenberg, J. G., Muzerie, C. J., et al. (1983). Candidate adenoviruses 40 and 41: Fastidious adenoviruses from human infant stool. Journal of Medical Virology, 11(3), 215–231.
Feeney, S. A., Armstrong, V. J., Mitchell, S. J., Crawford, L., McCaughey, C., & Coyle, P. V. (2011). Development and clinical validation of multiplex TaqMan® assays for rapid diagnosis of viral gastroenteritis. Journal of Medical Virology, 83, 1650–1656.
Fong, T. T., & Lipp, E. K. (2005). Enteric viruses of humans and animals in aquatic environments: Health risks, detection, and potential water quality assessment tools. Microbiology and Molecular Biology Reviews, 69(2), 357–371.
Fong, T. T., Phanikumar, M. S., Xagoraraki, I., & Rose, J. B. (2010). Quantitative detection of human adenoviruses in wastewater and combined sewer overflows influencing a Michigan river. Applied and Environmental Microbiology, 76, 715–723.
Francois, P., Tangomo, M., Hibbs, J., Bonetti, E. J., Boehme, C. C., Notomi, T., et al. (2011). Robustness of a loop-mediated isothermal amplification reaction for diagnostic applications. FEMS Immunology and Medical Microbiology, 62(1), 41–48.
Haramoto, E., Katayama, H., Oguma, K., & Ohgaki, S. (2007). Quantitative analysis of human enteric adenoviruses in aquatic environments. Journal of Applied Microbiology, 103, 2153–2159.
Haramoto, E., Kitajima, M., Katayama, H., & Ohgaki, S. (2010). Real-time PCR detection of adenoviruses, polyoma viruses, and torque teno viruses in river water in Japan. Water Research, 44, 1747–1752.
Harrach, B., Benkö, M., Both, G. W., Brown, M., Davison, A. J., Echavarria, M., et al. (2011). Family Adenoviridae. In A. M. Q. King, M. J. Adams, E. B. Carstens, & E. J. Lefkowitz (Eds.), Virus taxonomy: Classification and nomenclature of viruses. Ninth report of the international committee on taxonomy of viruses (pp. 125–141). San Diego: Elsevier.
He, J. W., & Jiang, S. (2005). Quantification of enterococci and human adenoviruses in environmental samples by real-time PCR. Applied and Environmental Microbiology, 71, 2250–2255.
Heim, A., Ebnet, C., Harste, G., & Pring-Akerblom, P. (2003). Rapid and quantitative detection of human adenovirus DNA by real-time PCR. Journal of Medical Virology, 70(2), 228–239.
Hernroth, B. E., Conden-Hansson, A. C., Rehnstam-Holm, A. S., Girones, R., & Allard, A. K. (2002). Environmental factors influencing human viral pathogens and their potential indicator organisms in the blue mussel, Mytilus edulis: The first Scandinavian report. Applied and Environmental Microbiology, 68, 4523–4533.
Higgins, R. R., Beniprashad, M., Cardona, M., Masney, S., Low, D. E., & Gubbay, J. B. (2011). Evaluation and verification of the Seeplex Diarrhea-V ACE assay for simultaneous detection of adenovirus, rotavirus, and norovirusgenogroups I and II in clinical stool specimens. Journal of Clinical Microbiology, 49(9), 3154–3162.
Hoorfar, J., Cook, N., Malorny, B., Wagner, M., De Medici, D., Abdulmawjood, A., & Fach, P. (2004). Diagnostic PCR: Making internal amplification control mandatory. Letters in Applied Microbiology, 38(2), 79–80.
Jex, A. R., Stanley, K. K., Lo, W., Littman, R., Verweij, J. J., Campbell, B. E., et al. (2012). Detection of diarrhoeal pathogens in human faeces using an automated, robotic platform. Molecular and Cellular Probes, 26(1), 11–15.
Jiang, S., Dezfulian, H., & Chu, W. (2005). Real-time quantitative PCR for enteric adenovirus serotype 40 in environmental waters. Canadian Journal of Microbiology, 51(5), 393–398.
Jothikumar, N., Cromeans, T. L., Hill, V. R., Lu, X., Sobsey, M. D., & Erdman, D. D. (2005). Quantitative real-time PCR assays for detection of human adenoviruses and identification of serotypes 40 and 41. Applied and Environmental Microbiology, 71, 3131–3136.
Kidd, A. H., Banatvala, J. E., & de Jong, J. C. (1983). Antibodies to fastidious faecal adenoviruses (species 40 and 41) in sera from children. Journal of Medical Virology, 11(4), 333–341.
Kim, J. M., Kim, S. Y., Park, Y. B., Kim, H. J., Min, B. S., Cho, J. C., et al. (2012). Simultaneous detection of major enteric viruses using a combimatrix microarray. Journal of Microbiology, 50(6), 970–977.
Kokkinos, P. A., Ziros, P. G., Bellou, M., & Vantarakis, A. (2014). Loop-mediated isothermal amplification (LAMP) for the detection of Salmonella in food. Food Analytical Methods, 7, 512–526.
Kokkinos, P. A., Ziros, P. G., Monini, M., Lampropoulou, P., Karampini, A., Papachatzi, E., et al. (2013). Rare types of Rotaviruses isolated from children with acute gastroenteritis in Patras. Greece. Intervirology, 56(4), 237–241.
Kokkinos, P. A., Ziros, P. G., Mpalasopoulou, A., Galanis, A., & Vantarakis, A. (2011). Molecular detection of multiple viral targets in untreated urban sewage from Greece. Virology Journal, 27(8), 195.
Lee, S. H., & Kim, S. J. (2002). Detection of infectious enteroviruses and adenoviruses in tap water in urban areas in Korea. Water Research, 36(1), 248–256.
Lee, J. I., Lee, G. C., Chung, J. Y., Han, T. H., Lee, Y. K., Kim, M. S., & Lee, C. H. (2012). Detection and molecular characterization of adenoviruses in Korean children hospitalized with acute gastroenteritis. Microbiology and Immunology, 56(8), 523–528.
Leung, T. K., & Brown, M. (2011). Block in entry of enteric adenovirus type 41 in HEK293 cells. Virus Research, 156, 54–63.
Liu, J., Kibiki, G., Maro, V., Maro, A., Kumburu, H., Swai, N., et al. (2011). Multiplex reverse transcription PCR Luminex assay for detection and quantitation of viral agents of gastroenteritis. Journal of Clinical Virology, 50(4), 308–313.
Logan, C., O’Leary, J. J., & O’Sullivan, N. (2006). Real-time reverse transcription-PCR for detection of rotavirus and adenovirus as causative agents of acute viral gastroenteritis in children. Journal of Clinical Microbiology, 44(9), 3189–3195.
Mellou, K., Katsioulis, A., Potamiti-Komi, M., Pournaras, S., Kyritsi, M., Katsiaflaka, A., et al. (2013a). A large waterborne gastroenteritis outbreak in central Greece, March 2012: Challenges for the investigation and management. Epidemiology and Infection, 30, 1–11.
Mellou, K., Sideroglou, T., Potamiti-Komi, M., Kokkinos, P., Ziros, P., Georgakopoulou, T., & Vantarakis, A. (2013b). Epidemiological investigation of two parallel gastroenteritis outbreaks in school settings. BMC Public Health, 13, 241.
Myrmel, M., Berg, E. M., Rimstad, E., & Grinde, B. (2004). Detection of enteric viruses in shellfish from the Norwegian coast. Applied and Environmental Microbiology, 70(5), 2678–2684.
Nakamura, M., Hirano, E., Kowada, K., Ishiguro, F., Yamagishi, Z., Adhikary, A. K., et al. (2012). Surveillance of adenovirus D in patients with epidemic keratoconjunctivitis from Fukai Prefecture, Japan, 1995-2010. Journal of Medical Virology, 84(1), 81–86.
Navidad, J. F., Griswold, D. J., Gradus, M. S., & Bhattacharyya, S. (2013). Evaluation of Luminex xTAG gastrointestinal pathogen analyte-specific reagents for high-throughput, simultaneous detection of bacteria, viruses, and parasites of clinical and public health importance. Journal of Clinical Microbiology, 51(9), 3018–3024.
Noble, R. T., Allen, S. M., Blackwood, A. D., Chu, W., Jiang, S. C., Lovelace, G. L., et al. (2003). Use of viral pathogens and indicators to differentiate between human and non-human fecal contamination in a microbial course tracking comparison study. Journal of Water and Health, 1, 195–207.
Notomi, T., Okayama, H., Masubuchi, H., Yonekawa, T., Watanabe, K., Amino, N., & Hase, T. (2000). Loop-mediated isothermal amplification of DNA. Nucleic Acids Research, 28, e63.
Robinson, C. M., Singh, G., Lee, J. Y., Dehghan, S., Rajaiya, J., Liu, E. B., et al. (2013). Molecular evolution of human adenoviruses. Scientific Reports, 3, 1812.
Rodríguez-Lázaro, D., Cook, N., Ruggeri, F. M., Sellwood, J., Nasser, A., Nascimento, M. S., et al. (2012). Virus hazards from food, water and other contaminated environments. FEMS Microbiology Reviews, 36, 786–814.
Sdiri-Loulizi, K., Gharbi-Khelifi, H., de Rougemont, A., Hassine, M., Chouchane, S., Sakly, N., et al. (2009). Molecular epidemiology of human astrovirus and adenovirus serotypes 40/41 strains related to acute diarrhea in Tunisian children. Journal of Medical Virology, 81, 1895–1902.
Siqueira-Silva, J., Yeda, F. P., Favier, A. L., Mezin, P., Silva, M. L., Barrella, K. M., et al. (2009). Infection kinetics of human adenovirus serotype 41 in HEK 293 cells. Memórias do Instituto Oswaldo Cruz, 104, 736–744.
Techathuvanan, C., & D’Souza, D. H. (2012). Reverse-transcriptase loop-mediated isothermal amplification as a rapid screening/monitoring tool for Salmonella enterica detection in liquid whole eggs. Journal of Food Science, 77(4), M200–M205.
Tiemessen, C. T., & Nel, M. J. (1996). Detection and typing of subgroup F adenoviruses using the polymerase chain reaction. Journal of Virological Methods, 59(1–2), 73–82.
Van Heerden, J., Ehlers, M. M., Heim, A., & Grabow, W. O. (2005). Prevalence, quantification and typing of adenoviruses detected in river and treated drinking water in South Africa. Journal of Applied Microbiology, 99(2), 234–242.
Van Maarseveen, N. M., Wessels, E., de Brouwer, C. S., Vossen, A. C., & Claas, E. C. (2010). Diagnosis of viralgastro enteritis by simultaneous detection of Adenovirus group F, Astrovirus, Rotavirus group A, Norovirus genogroups I and II, and Sapovirus in two internally controlled multiplex real-time PCR assays. Journal of Clinical Virology, 49(3), 205–210.
Wessels, E., Rusman, L. G., van-Bussel, M. J., & Claas, E. C. (2013). Added value of multiplex Luminex Gastrointestinal Pathogen Panel (xTAG® GPP) testing in the diagnosis of infectious gastroenteritis. Clinical Microbiology and Infection,. doi:10.1111/1469-0691.12364.
Wyn-Jones, A. P., Carducci, A., Cook, N., D’Agostino, M., Divizia, M., Fleischer, J., et al. (2011). Surveillance of adenoviruses and noroviruses in European recreational waters. Water Research, 45, 1025–1038.
Yang, J. L., Ma, G. P., Yang, R., Yang, S. Q., Fu, L. Z., Cheng, A. C., et al. (2010). Simple and rapid detection of Salmonella serovar Enteritidis under field conditions by loop-mediated isothermal amplification. Journal of Applied Microbiology, 109(5), 1715–1723.
Ziros, P. G., Kokkinos, P. A., Leggaki, E., & Vantarakis, A. (2011). Development of an optimized method for the detection of airborne viruses with real-time PCR analysis. Virology Journal, 8, 369.
Author information
Authors and Affiliations
Corresponding author
Additional information
P. G. Ziros and P. A. Kokkinos have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ziros, P.G., Kokkinos, P.A., Allard, A. et al. Development and Evaluation of a Loop-Mediated Isothermal Amplification Assay for the Detection of Adenovirus 40 and 41. Food Environ Virol 7, 276–285 (2015). https://doi.org/10.1007/s12560-015-9182-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-015-9182-8