Summary
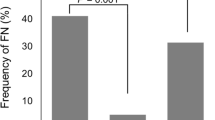
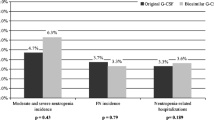
PURPOSE: Granulocyte-colony stimulating factors (G-CSFs) can effectively protect cancer patients receiving chemotherapy from neutropenic complications. To increase the efficacy, an individualised algorithm for the administration of G-CSF and anti-infectives was developed. Its impact on the neutropenic complications and entailed dose modifications in breast cancer patients receiving FEC-100 or TAC was evaluated. PATIENTS AND METHODS: Supportive therapy comprised G-CSF (filgrastim, lenograstim or pegfilgrastim), antibiotics and antimycotics. During each chemotherapy cycle, leukocyte/granulocyte counts were repeatedly evaluated and the type, dosing and application frequency of supportive therapies immediately adjusted as soon as the cell counts changed. Medical charts of early breast cancer patients who had received FEC-100 or TAC and supportive therapy according to the individualised protocol between 2004 and 2009, were retrospectively evaluated at the Oncology Department of the General Hospital Klagenfurt, Austria. RESULTS: Sixty-two FEC-100 and 56 TAC patients were evaluated. Of the 696 chemotherapy cycles, 693 included G-CSF support. Overall proportions of cycles with grade 4 neutropenia (FEC-100, 1.7%; TAC, 1.2%) and febrile neutropenia (FN) (FEC-100, 1.7%; TAC, 0.9%) as well as dose reductions (FEC-100, 0.6%; TAC, 1.5%) and delays (FEC-100, 4.2%; TAC, 5.1%) were very low. This was true also for elderly patients (>50 years). No patient developed FN in the first cycle. Although more toxic, TAC was associated with less neutropenic complications than FEC-100. CONCLUSION: Close-meshed monitoring of leukocyte/granulocyte counts and immediate adjustment of dosing and application frequencies of G-CSF and anti-infectives, also during the chemotherapy cycles, are highly effective in preventing neutropenic complications and their consequences in the clinical practice.
Similar content being viewed by others
Abbreviations
- CBC:
-
complete blood count
- CT:
-
chemotherapy
- ER:
-
oestrogen receptor
- FEC:
-
5-fluorouracil, epirubicin, cyclophosphamide
- FISH:
-
fluorescence in situ hybridisation
- FN:
-
febrile neutropenia
- Gc:
-
granulocytes
- G-CSF:
-
granulocyte colony-stimulating factor
- i.v.:
-
intravenous
- Lc:
-
leucocytes
- PR:
-
progesterone receptor
- TAC:
-
docetaxel, doxorubicin, cyclophosphamide
References
Trudeau M, Charbonneau F, Gelmon K, et al. Selection of adjuvant chemotherapy for treatment of node-positive breast cancer. Lancet Oncol, 6: 886–898, 2005
Bedard PL, Di Leo A, Piccart-Gebhart MJ. Taxanes: optimizing adjuvant chemotherapy for early-stage breast cancer. Nat Rev Clin Oncol, 7: 22–36, 2010
Smith TJ, Khatcheressian J, Lyman GH, et al. Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol, 24: 3187–3205, 2006
Aapro MS, Cameron DA, Pettengell R, et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer, 42: 2433–2453, 2006
Crawford J, Althaus B, Armitage J, et al. National comprehensive cancer network (NCCN). Myeloid growth factors. Clinical practice guidelines in oncology. J Natl Compr Canc Netw, 5: 188–202, 2007
Zielinski CC, Awada A, Cameron DA, et al. The impact of new European Organisation for Research and Treatment of Cancer guidelines on the use of granulocyte colony-stimulating factor on the management of breast cancer patients. Eur J Cancer, 44: 353–365, 2008
Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer, 100: 228–237, 2004
Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer, 106: 2258–2266, 2006
Leonard RCF, Miles D, Thomas R, et al. Impact of neutropenia on delivering planned adjuvant chemotherapy: UK audit of primary breast cancer patients. Br J Cancer, 89: 2062–2068, 2003
Pettengell R, Schwenkglenks M, Leonard R, et al. Neutropenia occurrence and predictors of reduced chemotherapy delivery: results from the INC-EU prospective observational European neutropenia study. Support Care Cancer, 16: 1299–1309, 2008
Bonadonna G, Valagussa P, Moliterni A, et al. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med, 332: 901–906, 1995
Chirivella I, Bermejo B, Insa A, et al. Optimal delivery of anthracycline-based chemotherapy in the adjuvant setting improves outcome of breast cancer patients. Breast Cancer Res Treat, 114: 479–484, 2009
Holmes FA, O'Shaughnessy JA, Vukelja S, et al. Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J Clin Oncol, 20: 727–731, 2002
Green MD, Koelbl H, Baselga J, et al. A randomized doubleblind multicenter phase III study of fixed-dose single administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol, 14: 29–35, 2003
Gisselbrecht C, Haioun C, Lepage E, et al. Placebo-controlled phase III study of lenograstim (glycosylated recombinant human granulocyte colony-stimulating factor) in aggressive non-Hodgkin's lymphoma: factors influencing chemotherapy administration. Groupe d'Etude des Lymphomes de l'Adulte. Leuk Lymphoma, 25: 289–300, 1997
Pettengell R, Gurney H, Radford JA, et al. Granulocyte colony-stimulating factor to prevent dose-limiting neutropenia in non-Hodgkin's lymphoma: a randomized controlled trial. Blood, 80: 1430–1436, 1992
Kuderer NM, Dale DC, Crawford J, et al. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol, 25: 3158–3167, 2007
Johnston E, Crawford J, Blackwell S, et al. Randomized, dose-escalation study of SD/01 compared with daily filgrastim in patients receiving chemotherapy. J Clin Oncol, 18: 2522–2528, 2000
Molineux G, Kinstler O, Briddell B, et al. A new form of filgrastim with sustained duration in vivo and enhanced ability to mobilize PBPC in both mice and humans. Exp Hematol, 27: 1724–1734, 1999
Roche H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: the FNCLCC PACS 01 trial. J Clin Oncol, 24: 5664–5671, 2006
Romieu G, Clemens M, Mahlberg R, et al. Pegfilgrastim supports delivery of FEC-100 chemotherapy in elderly patients with high risk breast cancer: a randomized phase 2 trial. Crit Rev Oncol Hematol, 64: 64–72, 2007
Repetto L, Biganzoli L, Koehne CH, et al. EORTC Cancer in the Elderly Task Force guidelines for the use of colony-stimulatingfactors in elderly patients with cancer. Eur J Cancer, 39: 2264–2272, 2003
Vogel CL, Wojtukiewicz MZ, Carroll RR, et al. First and subsequent cycle use of Pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo controlled phase III study. J Clin Oncol, 23: 1178–1184, 2005
Brugger W, Bacon P, Lawrinson S, et al. Neutrophil recovery in elderly breast cancer patients receiving adjuvant anthracycline-containing chemotherapy with pegfilgrastim support. Crit Rev Oncol Hematol, 72: 265–269, 2009
von Minckwitz G, Kümmel S, du Bois A, et al. Pegfilgrastim ± ciprofloxacin for primary prophylaxis with TAC (docetaxel/doxorubicin/cyclophosphamide) chemotherapy for breast cancer. Results from the GEPARTRIO study. Ann Oncol, 19: 292–298, 2008
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klocker, J., Schumer, J., Kanatschnig, M. et al. Very low rates of neutropenic complications and chemotherapy dose modifications in early breast cancer patients receiving adjuvant FEC-100 or TAC and an individualised G-CSF and anti-infective support: results of a retrospective chart review. memo 3, 123–128 (2010). https://doi.org/10.1007/s12254-010-0221-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-010-0221-8