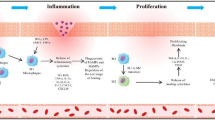
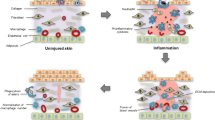
Abstract
The morphological and functional barriers caused by pathological scars are extremely painful for patients. Up to now, pathological scar poses a big unmet medical challenge for plastic surgeons and dermatologists worldwide. Macrophage polarization has shown a non-negligible effect on wound healing and scar formation. However, the role of macrophages in wound healing and pathological scar formation is still controversial. To summarize the latest data on probing biological functions of macrophage polarization in wound healing and scar formation and target macrophages in wound healing, we particularly paid attention to studies on different groups of macrophages, the transition among those groups, and modulators regulating the transition process. A comprehensive understanding of macrophage polarization in wound healing is certain to facilitate the development of new and efficient therapeutic modalities for pathological scar.
Similar content being viewed by others
References
NEGUT I, GRUMEZESCU V, GRUMEZESCU A. Treatment strategies for infected wounds [J]. Molecules, 2018, 23(9): 2392.
WANG G Q, XIA Z F. Monocyte subsets and their differentiation tendency after burn injury [J]. Frontiers of Medicine, 2013, 7(4): 397–400.
DI BENEDETTO P, RUSCITTI P, VADASZ Z, et al. Macrophages with regulatory functions, a possible new therapeutic perspective in autoimmune diseases [J]. Autoimmunity Reviews, 2019, 18(10): 102369.
TANG S, WAN M, HUANG W, et al. Maresins: Specialized proresolving lipid mediators and their potential role in inflammatory-related diseases [J]. Mediators of Inflammation, 2018, 2018: 2380319.
LIU L, MAO Y, XU B C, et al. Induction of neutrophil extracellular traps during tissue injury: Involvement of STING and Toll-like receptor 9 pathways [J]. Cell Proliferation, 2019, 52(3): e12579.
MANJILI F A, YOUSEFI-AHMADIPOUR A, ARABABADI M K. The roles played by TLR4 in the pathogenesis of multiple sclerosis; A systematic review article [J]. Immunology Letters, 2020, 220: 63–70.
HOU S, LIU Z, SHEN H, et al. Damage-associated molecular pattern-triggered immunity in plants [J]. Frontiers in Plant Science, 2019, 10: 646.
NA Y R, JE S, SEOK S H. Metabolic features of macrophages in inflammatory diseases and cancer [J]. Cancer Letters, 2018, 413: 46–58.
YAGÜE-CAPILLA M, GARCÍA-CABALLERO D, AGUILAR-PEREYRA F, et al. Base excision repair plays an important role in the protection against nitric oxide- and in vivo-induced DNA damage in Trypanosoma brucei [J]. Free Radical Biology and Medicine, 2019, 131: 59–71.
CAMPANA L, STARKEY LEWIS P J, PELLICORO A, et al. The STAT3-IL-10-IL-6 pathway is a novel regulator of macrophage efferocytosis and phenotypic conversion in sterile liver injury [J]. Journal of Immunology, 2018, 200(3): 1169–1187.
OISHI Y, MANABE I. Macrophages in inflammation, repair and regeneration [J]. International Immunology, 2018, 30(11): 511–528.
MARTINEZ F O, GORDON S. The M1 and M2 paradigm of macrophage activation: Time for reassessment [J]. F1000Prime Reports, 2014, 6: 13.
MANTOVANI A, SICA A, SOZZANI S, et al. The chemokine system in diverse forms of macrophage activation and polarization [J]. Trends in Immunology, 2004, 25(12): 677–686.
PEMMARI A, LEPPÄNEN T, PAUKKERI E L, et al. Attenuating effects of nortrachelogenin on IL-4 and IL-13 induced alternative macrophage activation and on bleomycin-induced dermal fibrosis [J]. Journal of Agricultural and Food Chemistry, 2018, 66(51): 13405–13413.
SU S, ZHAO Q, HE C, et al. miR-142-5p and miR-130a-3p are regulated by IL-4 and IL-13 and control profibrogenic macrophage program [J]. Nature Communications, 2015, 6: 8523.
WANG L X, ZHANG S X, WU H J, et al. M2b macrophage polarization and its roles in diseases [J]. Journal of Leukocyte Biology, 2019, 106(2): 345–358.
LAI Y S, PUTRA R B D S, AUI S P, et al. M2C polarization by baicalin enhances efferocytosis via upregulation of MERTK receptor [J]. The American Journal of Chinese Medicine, 2018, 46(8): 1899–1914.
LURIER E B, DALTON D, DAMPIER W, et al. Transcriptome analysis of IL-10-stimulated (M2c) macrophages by next-generation sequencing [J]. Immunobiology, 2017, 222(7): 847–856.
ROHANI M G, PARKS W C. Matrix remodeling by MMPs during wound repair [J]. Matrix Biology, 2015, 44/45/46: 113–121.
ARORA S, DEV K, AGARWAL B, et al. Macrophages: Their role, activation and polarization in pulmonary diseases [J]. Immunobiology, 2018, 223(4/5): 383–396.
GUO C, BURANYCH A, SARKAR D, et al. The role of tumor-associated macrophages in tumor vascularization [J]. Vascular Cell, 2013, 5(1): 20.
WYNN T A, VANNELLA K M. Macrophages in tissue repair, regeneration, and fibrosis [J]. Immunity, 2016, 44(3): 450–462.
ITALIANI P, MAZZA E M, LUCCHESI D, et al. Transcriptomic profiling of the development of the inflammatory response in human monocytes in vitro [J]. PLoS One, 2014, 9(2): e87680.
DAVID S, GREENHALGH A D, KRONER A. Macrophage and microglial plasticity in the injured spinal cord [J]. Neuroscience, 2015, 307: 311–318.
YU T, ZHAO L, HUANG X, et al. Enhanced activity of the macrophage M1/M2 phenotypes and phenotypic switch to M1 in periodontal infection [J]. Journal of Periodontology, 2016, 87(9): 1092–1102.
DALEY J M, BRANCATO S K, THOMAY A A, et al. The phenotype of murine wound macrophages [J]. Journal of Leukocyte Biology, 2010, 87(1): 59–67.
KIM H, WANG S Y, KWAK G, et al. Exosome-guided phenotypic switch of M1 to M2 macrophages for cutaneous wound healing [J]. Advanced Science, 2019, 6(20): 1900513.
KLUTH D C. Pro-resolution properties of macrophages in renal injury [J]. Kidney International, 2007, 72(3): 234–236.
MITCHELL S, THOMAS G, HARVEY K, et al. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: Stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo [J]. Journal of the American Society of Nephrology, 2002, 13(10): 2497–2507.
HU M S, WALMSLEY G G, BARNES L A, et al. Delivery of monocyte lineage cells in a biomimetic scaffold enhances tissue repair [J]. JCI Insight, 2017, 2(19): 96260.
CHEN L, DENG H, CUI H, et al. Inflammatory responses and inflammation-associated diseases in organs [J]. Oncotarget, 2018, 9(6): 7204–7218.
MIRZA R, DIPIETRO L A, KOH T J. Selective and specific macrophage ablation is detrimental to wound healing in mice [J]. The American Journal of Pathology, 2009, 175(6): 2454–2462.
ZHANG M Z, YAO B, YANG S, et al. CSF-1 signaling mediates recovery from acute kidney injury [J]. The Journal of Clinical Investigation, 2012, 122(12): 4519–4532.
LUCAS T, WAISMAN A, RANJAN R, et al. Differential roles of macrophages in diverse phases of skin repair [J]. Journal of Immunology, 2010, 184(7): 3964–3977.
HAMED S, BENNETT C L, DEMIOT C, et al. Erythropoietin, a novel repurposed drug: An innovative treatment for wound healing in patients with diabetes mellitus [J]. Wound Repair and Regeneration, 2014, 22(1): 23–33.
LEE J H, KAM E H, KIM S Y, et al. Erythropoietin attenuates postoperative cognitive dysfunction by shifting macrophage activation toward the M2 phenotype [J]. Frontiers in Pharmacology, 2017, 8: 839.
CALEY M P, MARTINS V L C, O’TOOLE E A. Metalloproteinases and wound healing [J]. Advances in Wound Care, 2015, 4(4): 225–234.
MADSEN D H, LEONARD D, MASEDUNSKAS A, et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway [J]. The Journal of Cell Biology, 2013, 202(6): 951–966.
WALMSLEY G G, MAAN Z N, WONG V W, et al. Scarless wound healing: Chasing the holy grail [J]. Plastic and Reconstructive Surgery, 2015, 135(3): 907–917.
BROWN J J, BAYAT A. Genetic susceptibility to raised dermal scarring [J]. British Journal of Dermatology, 2009, 161(1): 8–18.
ZHANG J L, QIAO Q, LIU M D, et al. IL-17 promotes scar formation by inducing macrophage infiltration [J]. The American Journal of Pathology, 2018, 188(7): 1693–1702.
GOREN I, ALLMANN N, YOGEV N, et al. A transgenic mouse model of inducible macrophage depletion: Effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes [J]. The American Journal of Pathology, 2009, 175(1): 132–147.
MCWHORTER F Y, DAVIS C T, LIU W F. Physical and mechanical regulation of macrophage phenotype and function [J]. Cellular and Molecular Life Sciences, 2015, 72(7): 1303–1316.
JAIN N, MOELLER J, VOGEL V. Mechanobiology of macrophages: How physical factors coregulate macrophage plasticity and phagocytosis [J]. Annual Review of Biomedical Engineering, 2019, 21: 267–297.
FENG Y, SUN Z L, LIU S Y, et al. Direct and indirect roles of macrophages in hypertrophic scar formation [J]. Frontiers in Physiology, 2019, 10: 1101.
MURRAY L A, ROSADA R, MOREIRA A P, et al. Serum amyloid P therapeutically attenuates murine bleomycin-induced pulmonary fibrosis via its effects on macrophages [J]. PLoS One, 2010, 5(3): e9683.
JIN Q, GUI L, NIU F, et al. Macrophages in keloid are potent at promoting the differentiation and function of regulatory T cells [J]. Experimental Cell Research, 2018, 362(2): 472–476.
KLINKERT K, WHELAN D, CLOVER A J P, et al. Selective M2 macrophage depletion leads to prolonged inflammation in surgical wounds [J]. European Surgical Research, 2017, 58(3/4): 109–120.
TANG P M, NIKOLIC-PATERSON D J, LAN H Y. Macrophages: Versatile players in renal inflammation and fibrosis [J]. Nature Reviews Nephrology, 2019, 15(3): 144–158.
DENG L, HUANG L, GUO Q Y, et al. CREB1 and Smad3 mediate TGF-β3-induced Smad7 expression in rat hepatic stellate cells [J]. Molecular Medicine Reports, 2017, 16(6): 8455–8462.
GIBBONS M A, MACKINNON A C, RAMACHANDRAN P, et al. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis [J]. American Journal of Respiratory and Critical Care Medicine, 2011, 184(5): 569–581.
WYNN T A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases [J]. The Journal of Clinical Investigation, 2007, 117(3): 524–529.
MORIKAWA M, DERYNCK R, MIYAZONO K. TGF-β and the TGF-β family: Context-dependent roles in cell and tissue physiology [J]. Cold Spring Harbor Perspectives in Biology, 2016, 8(5): a021873.
MENG X M, NIKOLIC-PATERSON D J, LAN H Y. TGF-β: The master regulator of fibrosis [J]. Nature Reviews Nephrology, 2016, 12(6): 325–338.
KLINKHAMMER B M, FLOEGE J, BOOR P. PDGF in organ fibrosis [J]. Molecular Aspects of Medicine, 2018, 62: 44–62.
FORCINA L, MIANO C, SCICCHITANO B M, et al. Signals from the niche: Insights into the role of IGF-1 and IL-6 in modulating skeletal muscle fibrosis [J]. Cells, 2019, 8(3): 232
SHI J, LI J, GUAN H, et al. Anti-fibrotic actions of interleukin-10 against hypertrophic scarring by activation of PI3K/AKT and STAT3 signaling pathways in scar-forming fibroblasts [J]. PLoS One, 2014, 9(5): e98228.
HE T, BAI X Z, JING J, et al. Notch signal deficiency alleviates hypertrophic scar formation after wound healing through the inhibition of inflammation [J]. Archives of Biochemistry and Biophysics, 2020, 682: 108286.
ARNO A I, GAUGLITZ G G, BARRET J P, et al. Up-to-date approach to manage keloids and hypertrophic scars: A useful guide [J]. Burns, 2014, 40(7): 1255–1266.
DARDENNE A D, WULFF B C, WILGUS T A. The alarmin HMGB-1 influences healing outcomes in fetal skin wounds [J]. Wound Repair and Regeneration, 2013, 21(2): 282–291.
LU S W, ZHANG X M, LUO H M, et al. Clodronate liposomes reduce excessive scar formation in a mouse model of burn injury by reducing collagen deposition and TGF-β1 expression [J]. Molecular Biology Reports, 2014, 41(4): 2143–2149.
ZHU Z S, DING J, MA Z S, et al. Systemic depletion of macrophages in the subacute phase of wound healing reduces hypertrophic scar formation [J]. Wound Repair and Regeneration, 2016, 24(4): 644–656.
BAECK C, WEI X, BARTNECK M, et al. Pharmacological inhibition of the chemokine C-C motif chemokine ligand 2 (monocyte chemoattractant protein 1) accelerates liver fibrosis regression by suppressing Ly-6C+ macrophage infiltration in mice [J]. Hepatology, 2014, 59(3): 1060–1072.
CHEN L, ZHOU X, FAN L. X, et al. Macrophage migration inhibitory factor promotes cyst growth in polycystic kidney disease [J]. The Journal of Clinical Investigation, 2015, 125(6): 2399–2412.
MURRAY L A, CHEN Q, KRAMER M S, et al. TGF-beta driven lung fibrosis is macrophage dependent and blocked by Serum amyloid P [J]. The International Journal of Biochemistry & Cell Biology, 2011, 43(1): 154–162.
UENO M, MAENO T, NISHIMURA S, et al. Alendronate inhalation ameliorates elastase-induced pulmonary emphysema in mice by induction of apoptosis of alveolar macrophages [J]. Nature Communications, 2015, 6: 6332.
WILLENBORG S, EMING S A. Cellular networks in wound healing [J]. Science, 2018, 362(6417): 891–892.
PEREZ-ASO M, CHIRIBOGA L, CRONSTEIN B N. Pharmacological blockade of adenosine A2A receptors diminishes scarring [J]. The FASEB Journal, 2012, 26(10): 4254–4263.
TREDGET E E, WANG R, SHEN Q, et al. Transforming growth factor-beta mRNA and protein in hypertrophic scar tissues and fibroblasts: Antagonism by IFN-alpha and IFN-gamma in vitro and in vivo [J]. Journal of Interferon & Cytokine Research, 2000, 20(2): 143–151.
DARAKHSHAN S, POUR A B. Tranilast: A review of its therapeutic applications [J]. Pharmacological Research, 2015, 91: 15–28.
WANG R, MAO Y, ZHANG Z, et al. Role of verapamil in preventing and treating hypertrophic scars and keloids [J]. International Wound Journal, 2016, 13(4): 461–468.
YANG S, HUANG S, FENG C, et al. Umbilical cord-derived mesenchymal stem cells: Strategies, challenges, and potential for cutaneous regeneration [J]. Frontiers of Medicine, 2012, 6(1): 41–47.
BAI D S, ZHAO Y, ZHU Q, et al. LZ205, a newly synthesized flavonoid compound, exerts antiinflammatory effect by inhibiting M1 macrophage polarization through regulating PI3K/AKT/mTOR signaling pathway [J]. Experimental Cell Research, 2018, 364(1): 84–94.
CAMILLE N, DEALTRY G. Regulation of M1/M2 macrophage polarization by Sutherlandia frutescens via NFκB and MAPK signaling pathways [J]. South African Journal of Botany, 2018, 116: 42–51.
JI J, XIANG P, LI T, et al. NOSH-NBP, a novel nitric oxide and hydrogen sulfide-releasing hybrid, attenuates ischemic stroke-induced neuroinflammatory injury by modulating microglia polarization [J]. Frontiers in Cellular Neuroscience, 2017, 11: 154.
DUGO L, BELLUOMO M G, FANALI C, et al. Effect of cocoa polyphenolic extract on macrophage polarization from proinflammatory M1 to antiinflammatory M2 state [J]. Oxidative Medicine and Cellular Longevity, 2017, 2017: 6293740.
BISSONNETTE E Y, PROULX L I, TURMEL V, et al. PCT-233, a novel modulator of pro- and anti-inflammatory cytokine production [J]. Clinical & Experimental Immunology, 2004, 135(3): 440–447.
SAKSIDA T, VUJICIC M, NIKOLIC I, et al. Compound A, a selective glucocorticoid receptor agonist, inhibits immunoinflammatory diabetes, induced by multiple low doses of streptozotocin in mice [J]. British Journal of Pharmacology, 2014, 171(24): 5898–5909.
CHANG Y, JIA X, WEI F, et al. CP-25, a novel compound, protects against autoimmune arthritis by modulating immune mediators of inflammation and bone damage [J]. Scientific Reports, 2016, 6: 26239.
ZHONG Y, CHIOU Y S, PAN M H, et al. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages [J]. Food Chemistry, 2012, 134(2): 742–748.
LIU J, LI K, ZHOU J, et al. Bisperoxovanadium induces M2-type macrophages and promotes functional recovery after spinal cord injury [J]. Molecular Immunology, 2019, 116: 56–62.
ZHANG Y K, WANG J, LIU L, et al. The effect of Lyciumbarbarum on spinal cord injury, particularly its relationship with M1 and M2 macrophage in rats [J]. BMC Complementary and Alternative Medicine, 2013, 13: 67.
LI D, LIU Q Y, SUN W, et al. 1,3,6,7-Tetrahydroxy-8-prenylxanthone ameliorates inflammatory responses resulting from the paracrine interaction of adipocytes and macrophages [J]. British Journal of Pharmacology, 2018, 175(10): 1590–1606.
LI T, PENG M Z, YANG Z Z, et al. 3D-printed IFN-γ-loading calcium silicate-β-tricalcium phosphate scaffold sequentially activates M1 and M2 polarization of macrophages to promote vascularization of tissue engineering bone [J]. Acta Biomaterialia, 2018, 71: 96–107.
FENG X J, QIN H H, SHI Q, et al. Chrysin attenuates inflammation by regulating M1/M2 status via activating PPARγ [J]. Biochemical Pharmacology, 2014, 89(4): 503–514.
XU G, FENG L L, SONG P P, et al. Isomeranzin suppresses inflammation by inhibiting M1 macrophage polarization through the NF-κBand ERKpathway [J]. International Immunopharmacology, 2016, 38: 175–185.
ZHANG X, XU F, LIU L, et al. (+)-Borneol improves the efficacy of edaravone against DSS-induced colitis by promoting M2 macrophages polarization via JAK2-STAT3 signaling pathway [J]. International Immunopharmacology, 2017, 53: 1–10.
PEI Z Y, WANG S H. Sevoflurane suppresses microglial M2 polarization [J]. Neuroscience Letters, 2017, 655: 160–165.
WEN M Y, YE J K, HAN Y L, et al. Hypertonic saline regulates microglial M2 polarization via miR-200b/KLF4 in cerebral edema treatment [J]. Biochemical and Biophysical Research Communications, 2018, 499(2): 345–353.
MEIRELES M, MARQUES C, NORBERTO S, et al. Anthocyanin effects on microglia M1/M2 phenotype: Consequence on neuronal fractalkine expression [J]. Behavioural Brain Research, 2016, 305: 223–228.
FENG X J, WENG D, ZHOU F F, et al. Activation of PPARγ by a natural flavonoid modulator, apigenin ameliorates obesity-related inflammation via regulation of macrophage polarization [J]. EBioMedicine, 2016, 9: 61–76.
YU X M, XU M Y, LI N, et al. β-elemene inhibits tumor-promoting effect of M2 macrophages in lung cancer [J]. Biochemical and Biophysical Research Communications, 2017, 490(2): 514–520.
YANG X D, XU S Q, QIAN Y W, et al. Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury [J]. Brain, Behavior, and Immunity, 2017, 64: 162–172.
IWANOWYCZ S, WANG J, ALTOMARE D, et al. Emodin bidirectionally modulates macrophage polarization and epigenetically regulates macrophage memory [J]. The Journal of Biological Chemistry, 2016, 291(22): 11491–11503.
LARA-GUZMAN O J, TABARES-GUEVARA J H, LEON-VARELA Y M, et al. Proatherogenic macrophage activities are targeted by the flavonoid quercetin [J]. The Journal of Pharmacology and Experimental Therapeutics, 2012, 343(2): 296–306.
CHAN K L, PILLON N J, SIVALOGANATHAN D M, et al. Palmitoleate reverses high fat-induced proinflammatory macrophage polarization via AMP-activated protein kinase (AMPK) [J]. Journal of Biological Chemistry, 2015, 290(27): 16979–16988.
ZHOU E S, LI Y M, YAO M J, et al. Niacin attenuates the production of pro-inflammatory cytokines in LPS-induced mouse alveolar macrophages by HCA2 dependent mechanisms [J]. International Immunopharmacology, 2014, 23(1): 121–126.
KANG C H, JAYASOORIYA R G P T, CHOI Y H, et al. β-Ionone attenuates LPS-induced proinflammatory mediators such as NO, PGE2 and TNF-α in BV2 microglial cells via suppression of the NF-κB and MAPK pathway [J]. Toxicology in Vitro, 2013, 27(2): 782–787.
LAN X, HAN X N, LI Q, et al. Pinocembrin protects hemorrhagic brain primarily by inhibiting toll-like receptor 4 and reducing M1 phenotype microglia [J]. Brain, Behavior, and Immunity, 2017, 61: 326–339.
TALMON M, ROSSI S, PASTORE A, et al. Vortioxetine exerts anti-inflammatory and immunomodulatory effects on human monocytes/macrophages [J]. British Journal of Pharmacology, 2018, 175(1): 113–124.
VELTMAN J D, LAMBERS M E, VAN NIMWEGEN M, et al. Zoledronic acid impairs myeloid differentiation to tumour-associated macrophages in mesothelioma [J]. British Journal of Cancer, 2010, 103(5): 629–641.
KIM S Y, MOON K A, JO H Y, et al. Antiinflammatory effects of apocynin, an inhibitor of NADPH oxidase, in airway inflammation [J]. Immunology and Cell Biology, 2012, 90(4): 441–448.
HART P H, BRAND C, CARSON C F, et al. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes [J]. Inflammation Research, 2000, 49(11): 619–626.
NOGUEIRA M N M, AQUINO S G, ROSSA JUNIOR C, et al. Terpinen-4-ol and alpha-terpineol (tea tree oil components) inhibit the production of IL-1β, IL-6 and IL-10 on human macrophages [J]. Inflammation Research, 2014, 63(9): 769–778.
PARK H Y, HAN M H, PARK C, et al. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells [J]. Food and Chemical Toxicology, 2011, 49(8): 1745–1752.
LI L, HAMILTON R F, TAYLOR D E, et al. Acrolein-induced cell death in human alveolar macrophages [J]. Toxicology and Applied Pharmacology, 1997, 145(2): 331–339.
KOHNO K, MIYAKE M, SANO O, et al. Anti-inflammatory and immunomodulatory properties of 2-amino-3H-phenoxazin-3-one [J]. Biological & Pharmaceutical Bulletin, 2008, 31(10): 1938–1945.
DONG R, GONG Y L, MENG W, et al. The involvement of M2 macrophage polarization inhibition in fenretinide-mediated chemopreventive effects on colon cancer [J]. Cancer Letters, 2017, 388: 43–53.
GAO S S, ZHOU J, LIU N, et al. Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13 [J]. Journal of Molecular and Cellular Cardiology, 2015, 85: 131–139.
JANG H M, KANG G D, VAN LE T K, et al. 4-Methoxylonchocarpin attenuates inflammation by inhibiting lipopolysaccharide binding to Toll-like receptor of macrophages and M1 macrophage polarization [J]. International Immunopharmacology, 2017, 45: 90–97.
SHI H, ZHENG K, SU Z L, et al. Sinomenine enhances microglia M2 polarization and attenuates inflammatory injury in intracerebral hemorrhage [J]. Journal of Neuroimmunology, 2016, 299: 28–34.
FENG L L, SONG P P, ZHOU H, et al. Pentamethoxyflavanone regulates macrophage polarization and ameliorates sepsis in mice [J]. Biochemical Pharmacology, 2014, 89(1): 109–118.
LU H, WU L F, LIU L P, et al. Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization [J]. Biochemical Pharmacology, 2018, 154: 203–212.
DONG J, ZHANG X, ZHANG L, et al. Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: A mechanism including AMPKα1/SIRT1 [J]. Journal of Lipid Research, 2014, 55(3): 363–374.
KIM Y J, PARK W. Anti-inflammatory effect of quercetin on RAW 264.7 mouse macrophages induced with polyinosinic-polycytidylic acid [J]. Molecules, 2016, 21(4): 450.
FU J, HUANG J J, LIN M, et al. Quercetin promotes diabetic wound healing via switching macrophages from M1 to M2 polarization [J]. Journal of Surgical Research, 2020, 246: 213–223.
SU F, YI H, XU L, et al. Fluoxetine and S-citalopram inhibit M1 activation and promote M2 activation of microglia in vitro [J]. Neuroscience, 2015, 294: 60–68.
LKHAGVAA B, TANI K J, SATO K, et al. Bestatin, an inhibitor for aminopeptidases, modulates the production of cytokines and chemokines by activated monocytes and macrophages [J]. Cytokine, 2008, 44(3): 386–391.
SOLANKI P, AMINOSHARIAE A, JIN G, et al. The effect of docosahexaenoic acid (DHA) on expression of IL-1β, IL-6, IL-8, and TNF-α in normal and lipopolysaccharide (LPS)-stimulated macrophages [J]. Quintessence International, 2013, 44(6): 393.
JUNG W K, LEE D Y, PARK C, et al. Cilostazol is anti-inflammatory in BV2 microglial cells by inactivating nuclear factor-kappaB and inhibiting mitogen-activated protein kinases [J]. British Journal of Pharmacology, 2010, 159(6): 1274–1285.
QIN C, FAN W H, LIU Q, et al. Fingolimod protects against ischemic white matter damage by modulating microglia toward M2 polarization via STAT3 pathway [J]. Stroke, 2017, 48(12): 3336–3346.
URBÁSKOVÁ P, ÁNDELOVÁ A, TORSOVÁ T, et al. Serratia marcescens as a cause of nosocomial infection in an intensive care unit [J]. VnitrniLekarstvi, 1978, 24(3): 254–259.
MALEK N, POPIOLEK-BARCZYK K, MIKA J, et al. Anandamide, acting via CB2 receptors, alleviates LPS-induced neuroinflammation in rat primary microglial cultures [J]. Neural Plasticity, 2015, 2015: 130639.
SU W J, ZHANG T, JIANG C L, et al. Clemastine alleviates depressive-like behavior through reversing the imbalance of microglia-related pro-inflammatory state in mouse hippocampus [J]. Frontiers in Cellular Neuroscience, 2018, 12: 412.
FENG Q, XU M, YU Y Y, et al. High-dose dexamethasone or all-trans-retinoic acid restores the balance of macrophages towards M2 in immune thrombocytopenia [J]. Journal of Thrombosis and Haemostasis, 2017, 15(9): 1845–1858.
JANG C H, CHOI J H, BYUN M S, et al. Chloroquine inhibits production of TNF-α, IL-1β and IL-6 from lipopolysaccharide-stimulated human mono-cytes/macrophages by different modes [J]. Rheumatology, 2006, 45(6): 703–710.
SONG Y X, DOU H, GONG W, et al. Bis-N-norgliovictin, a small-molecule compound from marine fungus, inhibits LPS-induced inflammation in macrophages and improves survival in sepsis [J]. European Journal of Pharmacology, 2013, 705(1/2/3): 49–60.
ARYANPOUR R, PASBAKHSH P, ZIBARA K, et al. Progesterone therapy induces an M1 to M2 switch in microglia phenotype and suppresses NLRP3 inflammasome in a cuprizone-induced demyelination mouse model [J]. International Immunopharmacology, 2017, 51: 131–139.
LIU X, WEN S, YAN F, et al. Salidroside provides neuroprotection by modulating microglial polarization after cerebral ischemia [J]. Journal of Neuroinflammation, 2018, 15(1): 39.
HE L, MARNEROS A G. Doxycycline inhibits polarization of macrophages to the proangiogenic M2-type and subsequent neovascularization [J]. The Journal of Biological Chemistry, 2014, 289(12): 8019–8028.
ZHU Y S, LI X Q, CHEN J Q, et al. The pentacyclic triterpene Lupeol switches M1 macrophages to M2 and ameliorates experimental inflammatory bowel disease [J]. International Immunopharmacology, 2016, 30: 74–84.
ZHU W, JIN Z S, YU J B, et al. Baicalin ameliorates experimental inflammatory bowel disease through polarization of macrophages to an M2 phenotype [J]. International Immunopharmacology, 2016, 35: 119–126.
KANG S, PARK S J, LEE A Y, et al. Ginsenoside Rg3 promotes inflammation resolution through M2 macrophage polarization [J]. Journal of Ginseng Research, 2018, 42(1): 68–74.
NEOG M K, SULTANA F, RASOOL M. Targeting RAW 264.7 macrophages (M1 type) with Withaferin-A decorated mannosylated liposomes induces repolarization via downregulation of NF-κB and controlled elevation of STAT-3 [J]. International Immunopharmacology, 2018, 61: 64–73.
WANG S X, WANG F J, YANG H Y, et al. Diosgenin glucoside provides neuroprotection by regulating microglial M1 polarization [J]. International Immunopharmacology, 2017, 50: 22–29.
KO H J, LO C Y, WANG B J, et al. Theaflavin-3, 3’-digallate, a black tea polyphenol, attenuates adipocyte-activated inflammatory response of macrophage associated with the switch of M1/M2-like phenotype [J]. Journal of Functional Foods, 2014, 11: 36–48.
LUO X Q, LI A, YANG X, et al. Paeoniflorin exerts neuroprotective effects by modulating the M1/M2 subset polarization of microglia/macrophages in the hippocampal CA1 region of vascular dementia rats via cannabinoid receptor 2 [J]. Chinese Medicine, 2018, 13: 14.
AMANTEA D, CERTO M, PETRELLI F, et al. Azithromycin protects mice against ischemic stroke injury by promoting macrophage transition towards M2 phenotype [J]. Experimental Neurology, 2016, 275: 116–125.
PAN J, JIN J L, GE H M, et al. Malibatol A regulates microglia M1/M2 polarization in experimental stroke in a PPARκ-dependent manner [J]. Journal of Neuroinflammation, 2015, 12: 1–11.
LIU X X, LI J, PENG X H, et al. Geraniin inhibits LPS-induced THP-1 macrophages switching to M1 phenotype via SOCS1/NF-κB pathway [J]. Inflammation, 2016, 39(4): 1421–1433.
PLASTIRA I, BERNHART E, GOERITZER M, et al. 1-Oleyl-lysophosphatidic acid (LPA) promotes polarization of BV-2 and primary murine microglia towards an M1-like phenotype [J]. Journal of Neuroinflammation, 2016, 13(1): 205.
ZHANG X, ZHOU M, GUO Y, et al. 1,25-dihydroxyvitamin D3 promotes high glucose-induced M1 macrophage switching to M2 via the VDRPPARγ signaling pathway [J]. BioMed Research International, 2015, 2015: 157834.
FIORCARI S, MAFFEI R, AUDRITO V, et al. Ibrutinib modifies the function of monocyte/macrophage population in chronic lymphocytic leukemia [J]. Oncotarget, 2016, 7(40): 65968–65981.
ROSENSON R S, TANGNEY C C, CASEY L C. Inhibition of proinflammatory cytokine production by pravastatin [J]. The Lancet, 1999, 353(9157): 983–984.
JUNG S, SIGLIENTI I, GRAUER O, et al. Induction of IL-10 in rat peritoneal macrophages and dendritic cells by glatiramer acetate [J]. Journal of Neuroimmunology, 2004, 148(1/2): 63–73.
JIANG M, LIU X H, ZHANG D H, et al. Celastrol treatment protects against acute ischemic stroke-induced brain injury by promoting an IL-33/ST2 axis-mediated microglia/macrophage M2 polarization [J]. Journal of Neuroinflammation, 2018, 15(1): 1–12.
GARCÍA J E L, RODRÍGUEZ F M, LÓPEZ A J, et al. Effect of cyclosporin A on inflammatory cytokine production by human alveolar macrophages [J]. Respiratory Medicine, 1998, 92(5): 722–728.
SCHILLING E, WEISS R, GRAHNERT A, et al. Molecular mechanism of LPS-induced TNF-α biosynthesis in polarized human macrophages [J]. Molecular Immunology, 2018, 93: 206–215.
GENSEL J C, ZHANG B. Macrophage activation and its role in repair and pathology after spinal cord injury [J]. Brain Research, 2015, 1619: 1–11.
HALSTEAD E S, UMSTEAD T M, DAVIES M L, et al. GM-CSF overexpression after influenza a virus infection prevents mortality and moderates M1-like airway monocyte/macrophage polarization [J]. Respiratory Research, 2018, 19(1): 3.
MANTOVANI A, VECCHI A, ALLAVENA P. Pharmacological modulation of monocytes and macrophages [J]. Current Opinion in Pharmacology, 2014, 17: 38–44.
HAMZEI TAJ S, LE BLON D, HOORNAERT C, et al. Targeted intracerebral delivery of the anti-inflammatory cytokine IL13 promotes alternative activation of both microglia and macrophages after stroke [J]. Journal of Neuroinflammation, 2018, 15(1): 174.
FERNANDO M R, REYES J L, IANNUZZI J, et al. The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages [J]. PLoS One, 2014, 9(4): e94188.
KUROWSKA-STOLARSKA M, STOLARSKI B, KEWIN P, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation [J]. Journal of Immunology, 2009, 183(10): 6469–6477.
KOBORI T, HAMASAKI S, KITAURA A, et al. Interleukin-18 amplifies macrophage polarization and morphological alteration, leading to excessive angiogenesis [J]. Frontiers in Immunology, 2018, 9: 334.
LUO B, WANG J, LIU Z, et al. Phagocyte respiratory burst activates macrophage erythropoietin signalling to promote acute inflammation resolution [J]. Nature Communications, 2016, 7: 12177.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: the National Natural Science Foundation of China (No. 81701907), and the Pujiang Program of SSTC (No. 18PJ1407100)
Rights and permissions
About this article
Cite this article
Chen, L., Cheng, L., Chen, T. et al. Macrophage Polarization in Skin Wound Healing: Progress in Biology and Therapeutics. J. Shanghai Jiaotong Univ. (Sci.) 27, 264–280 (2022). https://doi.org/10.1007/s12204-021-2276-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12204-021-2276-6