Abstract
Introduction
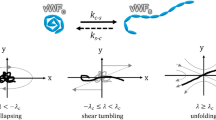
Pathological flows in patients with severe aortic stenosis are associated with acquired von Willebrand syndrome. This syndrome is characterized by excessive cleavage of von Willebrand factor by its main protease, A Disintegrin and Metalloproteinase with a Thrombospondin Type 1 Motif, Member 13 (ADAMTS13) leading to decreased VWF function and mucocutaneous bleeding. Aortic valve replacement and correction of the flow behavior to physiological levels reverses the syndrome, supporting the association between pathological flow and acquired von Willebrand syndrome. We investigated the effects of shear and elongational rates on von Willebrand factor cleavage in the presence of ADAMTS13.
Methods
We identified acquired von Willebrand syndrome in five patients with severe aortic stenosis. Doppler echography values from these patients were used to develop three computational fluid dynamic (CFD) aortic valve models (normal, mild and severe stenosis). Shear, elongational rates and exposure times identified in the CFD simulations were used as parameters for the design of microfluidic devices to test the effects of pathologic shear and elongational rates on the structure and function of von Willebrand factor.
Results
The shear rates (0–10,000s−1), elongational rates (0–1000 s−1) and exposure times (1–180 ms) tested in our microfluidic designs mimicked the flow features identified in patients with aortic stenosis. The shear and elongational rates tested in vitro did not lead to excessive cleavage or decreased function of von Willebrand factor in the presence of the protease.
Conclusions
High shear and elongational rates in the presence of ADAMTS13 are not sufficient for excessive cleavage of von Willebrand Factor.





Similar content being viewed by others
References
Amindari, A., L. Saltik, K. Kirkkopru, M. Yacoub, and H. C. Yalcin. Assessment of calcified aortic valve leaflet deformations and blood flow dynamics using fluid-structure interaction modeling. Inform. Med. Unlocked. 9:191–199, 2017.
Amindari, A., L. Saltik, K. Kirkkopru, M. Yacoub, and H. C. Yalcin. Informatics in medicine unlocked assessment of calcified aortic valve leaflet deformations and blood flow dynamics using fluid-structure interaction modeling. Inform. Med. Unlocked. 9(July):191–199, 2017.
Anderson, P. J., K. Kokame, and J. E. Sadler. Zinc and calcium ions cooperatively modulate ADAMTS13 activity. J Biol Chem. 281(2):850–857, 2006.
Baldauf, C., R. Schneppenheim, W. Stacklies, et al. Shear-induced unfolding activates von Willebrand factor A2 domain for proteolysis. J. Thromb. Haemost. 7(12):2096–2105, 2009.
Barker, A. J., P. van Ooij, K. Bandi, et al. Viscous energy loss in the presence of abnormal aortic flow. Magn. Reson. Med. 72(3):620–628, 2014.
Baumgartner, H., H. Kratzer, G. Helmreich, and P. Kuehn. Determination of aortic valve area by doppler echocardiography using the continuity equation: a critical evaluation. Cardiology. 77(2):101–111, 1990.
Bavo, A. M., G. Rocatello, F. Iannaccone, J. Degroote, J. Vierendeels, and P. Segers. Fluid-structure interaction simulation of prosthetic aortic valves: comparison between immersed boundary and arbitrary Lagrangian-Eulerian techniques for the mesh representation. PLoS ONE. 11(4):e0154517, 2016.
Blackshear, J. L., E. M. Wysokinska, R. E. Safford, et al. Indices of von Willebrand factor as biomarkers of aortic stenosis severity: (from the biomarkers of aortic stenosis severity [bass] study). Am. J. Cardiol. 111(3):374–381, 2013.
Blom, J. A. Monitoring of Respiration and Circulation. Boston: CRC Press, 2003.
Bluestein, D., and S. Einav. The effect of varying degrees of stenosis on the characteristics of turbulent pulsatile flow through heart valves. J. Biomech. 28(8):915–924, 1995.
Bluestein, D., L. Niu, R. T. Schoephoerster, and M. K. Dewanjee. Fluid mechanics of arterial stenosis: relationship to the development of mural thrombus. Ann. Biomed. Eng. 25(2):344, 1997.
Bortot, M., K. Ashworth, A. Sharifi, et al. Turbulent flow promotes cleavage of VWF (von Willebrand Factor) by ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type-1 motif, member 13). Arterioscler. Thromb. Vasc. Biol. 2019. https://doi.org/10.1161/ATVBAHA.119.312814.
Budde, U., R. Schneppenheim, J. Eikenboom, et al. Detailed von Willebrand factor multimer analysis in patients with von Willebrand disease in the European study, molecular and clinical markers for the diagnosis and management of type 1 von Willebrand disease (MCMDM-1VWD). J. Thromb. Haemost. 6(5):762–771, 2008.
Cao, W., S. Krishnaswamy, R. M. Camire, P. J. Lenting, and X. L. Zheng. Factor VIII accelerates proteolytic cleavage of von Willebrand factor by ADAMTS13. Proc. Natl. Acad. Sci. USA 105(21):7416–7421, 2008.
Chandra, S., N. M. Rajamannan, and P. Sucosky. Computational assessment of bicuspid aortic valve wall-shear stress: implications for calcific aortic valve disease. Biomech. Model Mechanobiol. 11(7):1085–1096, 2012.
Chen, D., C. S. Thomas, and J. L. Blackshear. Predictors of ccquired Von Willebrand syndrome in patients with aortic stenosis. Blood. 118(21):3318–3318, 2015.
Clendenen, N., A. Tollefson, M. Dzieciatkowska, et al. Correlation of pre-operative plasma protein concentrations in cardiac surgery patients with bleeding outcomes using a targeted quantitative proteomics approach. Proteomics Clin. Appl. 1:1, 2017. https://doi.org/10.1002/prca.201600175.
Crawley, J. T. B., R. de Groot, Y. Xiang, B. M. Luken, and D. A. Lane. Unraveling the scissile bond: how ADAMTS13 recognizes and cleaves von Willebrand factor. Blood. 118(12):3212–3221, 2011.
Dent, J. A., S. D. Berkowitz, J. Ware, C. K. Kasper, and Z. M. Ruggeri. Identification of a cleavage site directing the immunochemical detection of molecular abnormalities in type IIA von Willebrand factor. Proc. Natl. Acad. Sci. USA 87(16):6306–6310, 1990.
Dent, J. A., M. Galbusera, and Z. M. Ruggeri. Heterogeneity of plasma von willebrand factor multimers resulting from proteolysis of the constituent subunit. J. Clin. Invest. 88(3):774–782, 1991.
Feys, H. B., P. J. Anderson, K. Vanhoorelbeke, E. M. Majerus, and J. E. Sadler. Multi-step binding of ADAMTS13 to VWF. J. Thromb. Haemost. 2009. https://doi.org/10.1111/j.1538-7836.2009.03620.x.
Feys, H. B., P. J. Anderson, K. Vanhoorelbeke, E. M. Majerus, and J. E. Sadler. Multi-step binding of ADAMTS-13 to von Willebrand factor. J. Thromb. Haemost. 7(12):2088–2095, 2009.
Frank, R. D., R. Lanzmich, P. K. Haager, and U. Budde. Severe aortic valve stenosis: sustained cure of acquired von willebrand syndrome after surgical valve replacement. Clin. Appl. Thromb. 23(3):229–234, 2016.
Fu, H., Y. Jiang, D. Yang, F. Scheiflinger, W. P. Wong, and T. A. Springer. Flow-induced elongation of von Willebrand factor precedes tension-dependent activation. Nat. Commun. 8(1):324, 2017.
Grimard, B. H., and J. M. Larson. Aortic stenosis: diagnosis and treatment. Am. Fam. Physician. 78(6):717–724, 2008.
Ha, H., J. Lantz, M. Ziegler, et al. Estimating the irreversible pressure drop across a stenosis by quantifying turbulence production using 4D Flow MRI. Sci. Rep. 7:46618, 2017.
Han, Y., J. Xiao, E. Falls, and X. L. Zheng. A shear-based assay for assessing plasma ADAMTS13 activity and inhibitor in patients with thrombotic thrombocytopenic purpura. Transfusion. 51(7):1580–1591, 2011.
Jhun, C.-S., C. Siedlecki, L. Xu, et al. Stress and exposure time on von Willebrand factor degradation. Artif. Organs. 43(2):199–206, 2019.
Kleiman, N. S., and M. J. Reardon. Von Willebrand factor, paravalvular leak, and a new vista for TAVR. J. Thorac. Dis. 8(10):E1337–E1339, 2016.
Kokame, K., Y. Nobe, Y. Kokubo, A. Okayama, and T. Miyata. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br. J. Haematol. 129(1):93–100, 2005.
Larson, J. W., G. R. Yantz, Q. Zhong, et al. Single DNA molecule stretching in sudden mixed shear and elongational microflows. Lab. Chip. 6(9):1187–1199, 2006.
Lasne, D., C. Dey, M.-D. Dautzenberg, et al. Screening for von Willebrand disease: contribution of an automated assay for von Willebrand factor activity. Haemophilia 18(3):e158–e163, 2012.
Li, Y., K.-W. Hsiao, C. A. Brockman, et al. When ends meet: circular DNA stretches differently in elongational flows. Macromolecules. 48(16):5997–6001, 2015.
Majerus, E. M., P. J. Anderson, and J. E. Sadler. Binding of ADAMTS13 to von Willebrand Factor. J. Biol. Chem. 280(23):21773–21778, 2005.
Majerus, E. M., X. Zheng, E. A. Tuley, and J. E. Sadler. Cleavage of the ADAMTS13 propeptide is not required for protease activity. J. Biol. Chem. 278(47):46643–46648, 2003.
Maxwell, M. J., S. M. Dopheide, S. J. Turner, and S. P. Jackson. Shear induces a unique series of morphological changes in translocating platelets. Arterioscler. Thromb. Vasc. Biol. 26(3):663–669, 2006.
Meyer, D., and J. P. Girma. Von-willebrand-factor - structure and function. Thromb. Haemost. 70(1):99–104, 1993.
Morabito, M., C. Dong, W. Wei, et al. Internal tensile force and A2 domain unfolding of von Willebrand factor multimers in shear flow. Biophys. J. 115(10):1860–1871, 2018.
Neeves, K. B., S. F. Maloney, K. P. Fong, et al. Microfluidic focal thrombosis model for measuring murine platelet deposition and stability: PAR4 signaling enhances shear-resistance of platelet aggregates. J. Thromb. Haemost. 6(12):2193–2201, 2008.
Ng, C. J., K. R. McCrae, K. Ashworth, et al. Effects of anti-β2GPI antibodies on VWF release from human umbilical vein endothelial cells and ADAMTS13 activity. Res. Pract. Thromb. Haemost. 2(2):380–389, 2018.
Nishimura, R. A., C. M. Otto, R. O. Bonow, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 129(23):2440–2492, 2014.
Ober, T. J., S. J. Haward, C. J. Pipe, J. Soulages, and G. H. McKinley. Microfluidic extensional rheometry using a hyperbolic contraction geometry. Rheol. Acta. 52(6):529–546, 2013.
Sagheer, S., S. Rodgers, O. Yacoub, R. Dauer, S. Mcrae, and E. Duncan. Comparison of von Willebrand factor (VWF) activity levels determined by HemosIL AcuStar assay and HemosIL LIA assay with ristocetin cofactor assay by aggregometry. Haemophilia. 22(3):e200–e207, 2016.
Schneider, S. W., S. Nuschele, A. Wixforth, et al. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc. Natl. Acad. Sci. USA 104(19):7899–7903, 2007.
Sedaghat, A., H. Kulka, J.-M. Sinning, et al. Transcatheter aortic valve implantation leads to a restoration of von Willebrand factor (VWF) abnormalities in patients with severe aortic stenosis – Incidence and relevance of clinical and subclinical VWF dysfunction in patients undergoing transfemoral T. Thromb. Res. 151:23–28, 2017.
Sharifi, A., and H. Niazmand. Analysis of flow and LDL concentration polarization in siphon of internal carotid artery: non-Newtonian effects. Comput. Biol. Med. 65:93–102, 2015.
Shim, K., P. J. Anderson, E. A. Tuley, E. Wiswall, and Sadler. J. Evan. Platelet-VWF complexes are preferred substrates of ADAMTS13 under fluid shear stress. Blood. 111(2):651–657, 2008.
Siediecki, C. A., B. J. Lestini, K. K. Kottke-Marchant, S. J. Eppell, D. L. Wilson, and R. E. Marchant. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood. 88(8):2939–2950, 1996.
Sing, C. E., and A. Alexander-Katz. Elongational flow induces the unfolding of von Willebrand factor at physiological flow rates. Biophys. J. 98(9):L35–L37, 2010.
Singh, I., E. Themistou, L. Porcar, and S. Neelamegham. Fluid shear induces conformation change in human blood protein von willebrand factor in solution. Biophys. J. 96(6):2313–2320, 2009.
Skipwith, C. G., W. Cao, and X. L. Zheng. Factor VIII and platelets synergistically accelerate cleavage of von Willebrand factor by ADAMTS13 under fluid shear stress. J. Biol. Chem. 285(37):28596–28603, 2010.
Solomon, C., U. Budde, S. Schneppenheim, et al. Acquired type 2A von Willebrand syndrome caused by aortic valve disease corrects during valve surgery. Br. J. Anaesth. 106(4):494–500, 2011.
Sousa, P. C., F. T. Pinho, M. S. N. Oliveira, and M. A. Alves. Extensional flow of blood analog solutions in microfluidic devices. Biomicrofluidics. 5(1):14108, 2011.
South, K., B. M. Luken, J. T. B. Crawley, et al. Conformational activation of ADAMTS13. Proc. Natl. Acad. Sci. 111(52):18578–18583, 2014.
Spangenberg, T., U. Budde, D. Schewel, et al. Treatment of acquired von willebrand syndrome in aortic stenosis with transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 8(5):692–700, 2015.
Stockschlaeder, M., R. Schneppenheim, and U. Budde. Update on von Willebrand factor multimers: focus on high-molecular-weight multimers and their role in hemostasis. Blood Coagul. Fibrinolysis. 25(3):206–216, 2014.
Su, B., L. Zhong, X.-K. Wang, et al. Numerical simulation of patient-specific left ventricular model with both mitral and aortic valves by FSI approach. Comput. Methods Programs Biomed. 113(2):474–482, 2014.
Thubrikar, M. J. The Aortic Valve. London: Taylor & Francis, 1989.
Van Belle, E., A. Rauch, A. Vincentelli, et al. von Willebrand factor as a biological sensor of blood flow to monitor percutaneous aortic valve interventions. Circ. Res. 116(7):1193–1201, 2015.
Vincentelli, A., S. Susen, T. Le Tourneau, et al. Acquired von Willebrand Syndrome in Aortic Stenosis. N. Engl. J. Med. 349(4):343–349, 2003.
Xiang, Y., R. de Groot, J. T. B. Crawley, and D. A. Lane. Mechanism of von Willebrand factor scissile bond cleavage by a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13). Proc. Natl. Acad. Sci. 108(28):11602–11607, 2011.
Xu, A. J., and T. A. Springer. Calcium stabilizes the von Willebrand factor A2 domain by promoting refolding. Proc. Natl. Acad. Sci. USA 109(10):3742–3747, 2012.
Zhang, X., K. Halvorsen, C.-Z. Zhang, W. P. Wong, and T. A. Springer. Mechanoenzymatic cleavage of the ultralarge vascular protein von Willebrand Factor. Science (80-) 324(5932):1330–1334, 2009.
Zhou, M., X. Dong, C. Baldauf, et al. A novel calcium-binding site of von Willebrand factor A2 domain regulates its cleavage by ADAMTS13. Blood 117(17):4623–4631, 2011.
Zografos, K., F. Pimenta, M. A. Alves, and M. S. N. Oliveira. Microfluidic converging/diverging channels optimised for homogeneous extensional deformation. Biomicrofluidics 10(4):043508, 2016.
Funding
This work was supported in part by American Heart Association Pre-Doctoral Fellowship (18PRE33990253), the National Institutes of Health (R01 HL120728 and R01 HL141794) the National Science Foundation (1762705) and by an award from the American Heart Association (18CDA34110134).
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael R. King oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bortot, M., Sharifi, A., Ashworth, K. et al. Pathologic Shear and Elongation Rates Do Not Cause Cleavage of Von Willebrand Factor by ADAMTS13 in a Purified System. Cel. Mol. Bioeng. 13, 379–390 (2020). https://doi.org/10.1007/s12195-020-00631-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-020-00631-2