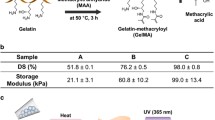
Abstract
Bioengineered hydrogels have been explored in cell and tissue engineering applications to support cell growth and modulate its behavior. A rationally designed scaffold should allow for encapsulated cells to survive, adhere, proliferate, remodel the niche, and can be used for controlled delivery of biomolecules. Here we report a microarray of composite bioadhesive microgels with modular dimensions, tunable mechanical properties and bulk modified adhesive biomolecule composition. Composite bioadhesive microgels of maleimide functionalized polyethylene glycol (PEG-MAL) with interpenetrating network (IPN) of gelatin ionically cross-linked with silicate nanoparticles were engineered by integrating microfabrication with Michael-type addition chemistry and ionic gelation. By encapsulating clinically relevant anchorage-dependent cervical cancer cells and suspension leukemia cells as cell culture models in these composite microgels, we demonstrate enhanced cell spreading, survival, and metabolic activity compared to control gels. The composite bioadhesive hydrogels represent a platform that could be used to study independent effect of stiffness and adhesive ligand density on cell survival and function. We envision that such microarrays of cell adhesive microenvironments, which do not require harsh chemical and UV crosslinking conditions, will provide a more efficacious cell culture platform that can be used to study cell behavior and survival, function as building blocks to fabricate 3D tissue structures, cell delivery systems, and high throughput drug screening devices.
Access this article
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.








Similar content being viewed by others
References
Alge, D. L., M. A. Azagarsamy, D. F. Donohue, and K. S. Anseth. Synthetically tractable click hydrogels for three-dimensional cell culture formed using tetrazine-norbornene chemistry. Biomacromolecules 14(4):949–953, 2013.
Allazetta, S., T. C. Hausherr, and M. P. Lutolf. Microfluidic synthesis of cell-type-specific artificial extracellular matrix hydrogels. Biomacromolecules 14(4):1122–1131, 2013.
Anseth, K. S., A. T. Metters, S. J. Bryant, P. J. Martens, J. H. Elisseeff, and C. N. Bowman. In situ forming degradable networks and their application in tissue engineering and drug delivery. J. Control Release 78(1–3):199–209, 2002.
Benton, J. A., C. A. DeForest, V. Vivekanandan, and K. S. Anseth. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng. Part A 15(11):3221–3230, 2009.
Benton, J. A., B. D. Fairbanks, and K. S. Anseth. Characterization of valvular interstitial cell function in three dimensional matrix metalloproteinase degradable PEG hydrogels. Biomaterials 30(34):6593–6603, 2009.
Burdick, J. A., and K. S. Anseth. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 23(22):4315–4323, 2002.
Chaudhuri, O., S. T. Koshy, C. Branco da Cunha, J. W. Shin, C. S. Verbeke, K. H. Allison, and D. J. Mooney. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014. doi:10.1038/nmat4009
Coyer, S. R., A. Singh, D. W. Dumbauld, D. A. Calderwood, S. W. Craig, E. Delamarche, and A. J. Garcia. Nanopatterning reveals an ECM area threshold for focal adhesion assembly and force transmission that is regulated by integrin activation and cytoskeleton tension. J. Cell Sci. 125(21):5110–5123, 2012.
DeForest, C. A., B. D. Polizzotti, and K. S. Anseth. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat. Mater. 8(8):659–664, 2009.
Dolatshahi-Pirouz, A., M. Nikkhah, A. K. Gaharwar, B. Hashmi, E. Guermani, H. Aliabadi, G. Camci-Unal, T. Ferrante, M. Foss, D. E. Ingber, and A. Khademhosseini. A combinatorial cell-laden gel microarray for inducing osteogenic differentiation of human mesenchymal stem cells. Sci. Rep. 4:3896, 2014.
Dumbauld, D. W., T. T. Lee, A. Singh, J. Scrimgeour, C. A. Gersbach, E. A. Zamir, J. P. Fu, C. S. Chen, J. E. Curtis, S. W. Craig, and A. J. Garcia. How vinculin regulates force transmission. Proc. Natl. Acad. Sci. USA 110(24):9788–9793, 2013.
Fairbanks, B. D., M. P. Schwartz, A. E. Halevi, C. R. Nuttelman, C. N. Bowman, and K. S. Anseth. A versatile synthetic extracellular matrix mimic via thiol-norbornene photopolymerization. Adv. Mater. 21(48):5005–5010, 2009.
Frampton, J. P., M. R. Hynd, M. L. Shuler, and W. Shain. Fabrication and optimization of alginate hydrogel constructs for use in 3D neural cell culture. Biomed. Mater. 6(1):015002, 2011.
Gaharwar, A. K., V. Kishore, C. Rivera, W. Bullock, C. J. Wu, O. Akkus, and G. Schmidt. Physically crosslinked nanocomposites from silicate-crosslinked PEO: mechanical properties and osteogenic differentiation of human mesenchymal stem cells. Macromol. Biosci. 12(6):779–793, 2012.
Gaharwar, A. K., S. M. Mihaila, A. Swami, A. Patel, S. Sant, R. L. Reis, A. P. Marques, M. E. Gomes, and A. Khademhosseini. Bioactive silicate nanoplatelets for osteogenic differentiation of human mesenchymal stem cells. Adv. Mater. 25(24):3329–3336, 2013.
Gaharwar, A. K., N. A. Peppas, and A. Khademhosseini. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 111(3):441–453, 2014.
Gaharwar, A. K., C. Rivera, C. J. Wu, B. K. Chan, and G. Schmidt. Photocrosslinked nanocomposite hydrogels from PEG and silica nanospheres: structural, mechanical and cell adhesion characteristics. Mater. Sci. Eng. C 33(3):1800–1807, 2013.
Headen, D. M., G. Aubry, H. Lu, and A. J. García. Microfluidic-based generation of size-controlled, biofunctionalized synthetic polymer microgels for cell encapsulation. Adv. Mater. 26(9):3003–3008, 2014.
Hiemstra, C., L. J. Aa, Z. Zhong, P. J. Dijkstra, and J. Feijen. Rapidly in situ-forming degradable hydrogels from dextran thiols through Michael addition. Biomacromolecules 8(5):1548–1556, 2007.
Hiemstra, C., L. J. van der Aa, Z. Zhong, P. J. Dijkstra, and J. Feijen. Novel in situ forming, degradable dextran hydrogels by Michael addition chemistry: synthesis, rheology, and degradation. Macromolecules 40(4):1165–1173, 2007.
Huebsch, N., P. R. Arany, A. S. Mao, D. Shvartsman, O. A. Ali, S. A. Bencherif, J. Rivera-Feliciano, and D. J. Mooney. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 9(6):518–526, 2010.
Hutson, C. B., J. W. Nichol, H. Aubin, H. Bae, S. Yamanlar, S. Al-Haque, S. T. Koshy, and A. Khademhosseini. Synthesis and characterization of tunable poly(ethylene glycol): gelatin methacrylate composite hydrogels. Tissue Eng. Part A 17(13–14):1713–1723, 2011.
Kesselman, L. R., S. Shinwary, P. R. Selvaganapathy, and T. Hoare. Synthesis of monodisperse, covalently cross-linked, degradable “smart” microgels using microfluidics. Small 8(7):1092–1098, 2012.
Kunz-Schughart, L. A., M. Kreutz, and R. Knuechel. Multicellular spheroids: a three-dimensional in vitro culture system to study tumour biology. Int. J. Exp. Pathol. 79(1):1–23, 1998.
Lee, G. Y., P. A. Kenny, E. H. Lee, and M. J. Bissell. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 4(4):359–365, 2007.
Lei, Y., S. Gojgini, J. Lam, and T. Segura. The spreading, migration and proliferation of mouse mesenchymal stem cells cultured inside hyaluronic acid hydrogels. Biomaterials 32(1):39–47, 2011.
Lim, F., and A. M. Sun. Microencapsulated islets as bioartificial endocrine pancreas. Science 210(4472):908–910, 1980.
Loessner, D., K. S. Stok, M. P. Lutolf, D. W. Hutmacher, J. A. Clements, and S. C. Rizzi. Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 31(32):8494–8506, 2010.
Lutolf, M. P., P. M. Gilbert, and H. M. Blau. Designing materials to direct stem-cell fate. Nature 462(7272):433–441, 2009.
Lutolf, M. P., and J. A. Hubbell. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 23(1):47–55, 2005.
Magley, J., C. Moyers, K. S. Ballard, and S. Tedjarati. Secondary cervical cancer in a patient with chronic lymphocytic leukemia and recurrent chronic lymphocytic leukemia mimicking recurrent cervical dysplasia: a case report. J. Reprod. Med. 55(3–4):175–178, 2010.
Metters, A., and J. Hubbell. Network formation and degradation behavior of hydrogels formed by Michael-type addition reactions. Biomacromolecules 6(1):290–301, 2005.
Miller, B. E., F. R. Miller, and G. H. Heppner. Factors affecting growth and drug sensitivity of mouse mammary tumor lines in collagen gel cultures. Cancer Res. 45(9):4200–4205, 1985.
Panda, P., S. Ali, E. Lo, B. G. Chung, T. A. Hatton, A. Khademhosseini, and P. S. Doyle. Stop-flow lithography to generate cell-laden microgel particles. Lab Chip 8(7):1056–1061, 2008.
Phelps, E. A., N. O. Enemchukwu, V. F. Fiore, J. C. Sy, N. Murthy, T. A. Sulchek, T. H. Barker, and A. J. Garcia. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv. Mater. 24(1):64–70, 2012.
Qiu, Y., J. J. Lim, L. Scott, Jr., R. C. Adams, H. T. Bui, and J. S. Temenoff. PEG-based hydrogels with tunable degradation characteristics to control delivery of marrow stromal cells for tendon overuse injuries. Acta Biomater. 7(3):959–966, 2011.
Raeber, G. P., M. P. Lutolf, and J. A. Hubbell. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys. J. 89(2):1374–1388, 2005.
Rossow, T., J. A. Heyman, A. J. Ehrlicher, A. Langhoff, D. A. Weitz, R. Haag, and S. Seiffert. Controlled synthesis of cell-laden microgels by radical-free gelation in droplet microfluidics. J. Am. Chem. Soc. 134(10):4983–4989, 2012.
Sala, A., P. Hanseler, A. Ranga, M. P. Lutolf, J. Voros, M. Ehrbar, and F. E. Weber. Engineering 3D cell instructive microenvironments by rational assembly of artificial extracellular matrices and cell patterning. Integr. Biol. (Camb.) 3(11):1102–1111, 2011.
Salimath, A. S., E. A. Phelps, A. V. Boopathy, P. L. Che, M. Brown, A. J. Garcia, and M. E. Davis. Dual delivery of hepatocyte and vascular endothelial growth factors via a protease-degradable hydrogel improves cardiac function in rats. PLoS ONE 7(11):e50980, 2012.
Selimovic, S., J. Oh, H. Bae, M. Dokmeci, and A. Khademhosseini. Microscale strategies for generating cell-encapsulating hydrogels. Polymers (Basel) 4(3):1554, 2012.
Singh, A., H. Qin, I. Fernandez, J. Wei, J. Lin, L. W. Kwak, and K. Roy. An injectable synthetic immune-priming center mediates efficient T-cell class switching and T-helper 1 response against B cell lymphoma. J. Controlled Release Off. J. Controlled Release Soc. 155(2):184–192, 2011.
Singh, A., S. Suri, T. Lee, J. M. Chilton, M. T. Cooke, W. Chen, J. Fu, S. L. Stice, H. Lu, T. C. McDevitt, and A. J. Garcia. Adhesion strength-based, label-free isolation of human pluripotent stem cells. Nat. Methods 10(5):438–444, 2013.
Singh, A., S. Suri, and K. Roy. In-situ crosslinking hydrogels for combinatorial delivery of chemokines and siRNA-DNA carrying microparticles to dendritic cells. Biomaterials 2009. doi:10.1016/j.biomaterials.2009.06.001.
Suri, S., and C. E. Schmidt. Photopatterned collagen-hyaluronic acid interpenetrating polymer network hydrogels. Acta Biomater. 5(7):2385–2397, 2009.
Tomei, A. A., S. Siegert, M. R. Britschgi, S. A. Luther, and M. A. Swartz. Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J. Immunol. 183(7):4273–4283, 2009.
Weaver, V. M., S. Lelievre, J. N. Lakins, M. A. Chrenek, J. C. Jones, F. Giancotti, Z. Werb, and M. J. Bissell. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell 2(3):205–216, 2002.
Weaver, V. M., O. W. Petersen, F. Wang, C. A. Larabell, P. Briand, C. Damsky, and M. J. Bissell. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 137(1):231–245, 1997.
Acknowledgments
The authors would like to acknowledge financial support by Grants from the National Institutes of Health (1R21CA185236-01) and the Cornell University-Ithaca and Weill Cornell Medical College seed grant. The authors also thank Prof. Marjolein C.H. van der Meulen in the Department of Biomedical Engineering and the Sibley School of Mechanical and Aerospace Engineering for providing cells. The authors also thank Dr. Brian Kirby in Sibley School of Mechanical and Aerospace Engineering at Cornell University for access to the cells, microscopy facility and spectrophotometer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Conflict of interest
Ravi Ghanshyam Patel, Alberto Purwada, Leandro Cerchietti, Giorgio Inghirami, Ari Melnick, Akhilesh Gaharwar, and Ankur Singh declare that they have no conflicts of interest.
Ethical standards
No animal or human studies were carried out by the authors for this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor David Mooney oversaw the review of this article.
This paper is part of the 2014 Young Innovators Issue.
Ankur Singh is an Assistant Professor in the Sibley School of Mechanical & Aerospace Engineering at Cornell University, where he directs the Immunotherapy and Cell Engineering Laboratory. Dr. Singh has expertise in the engineering of biomaterials based platforms for immune cell modulation, cell-biomaterial interactions, cell adhesion, nanoengineered materials, and stem cells. He received his postdoctoral training at Georgia Institute of Technology where he employed engineering and molecular cell biology principles to understand force response and mechanotransduction during the process of human stem cell reprogramming and differentiation. Dr. Singh received his Ph.D. in Biomedical Engineering at The University of Texas at Austin. His research has established multi-modal, synthetic immune priming centers and micro-nanoparticle vaccines for blood cancer and viral infections. His other relevant work includes developing self-assembly nanogels for protein therapeutic delivery to reduce osteoarthritis associated inflammation in knee joints. He has published peer-reviewed paper in Nature Methods, PNAS, Advanced Materials, Molecular Therapy, Biomaterials, Journal of Controlled Release, and Journal of Cell Science. His research is supported by the National Institutes of Health and National Cancer Institute.

Rights and permissions
About this article
Cite this article
Patel, R.G., Purwada, A., Cerchietti, L. et al. Microscale Bioadhesive Hydrogel Arrays for Cell Engineering Applications. Cel. Mol. Bioeng. 7, 394–408 (2014). https://doi.org/10.1007/s12195-014-0353-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-014-0353-8