Abstract
Background and aims
Many models have been developed to predict liver-related events (LRE) in chronic hepatitis B, few focused on compensated HBV-induced cirrhosis. We aimed to describe the incidence of LRE and to determine independent risk predictors of LRE in compensated HBV-induced cirrhosis patients receiving antiviral therapy using routinely available parameters.
Methods
Prospective cohorts of treatment-naïve adults with compensated HBV-induced cirrhosis were enrolled. Patients were treated with entecavir (ETV) or ETV + thymosin-alpha1 (Thy-α1) or lamivudine (LAM) + adefovir (ADV). Data were collected at baseline and every 6 months. LRE was defined as development of decompensation, HCC or death.
Results
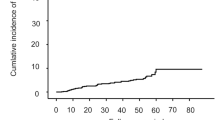
Totally 937 patients were included, 608 patients treated with ETV, 252 with ETV + Thy-α1, and 77 with LAM + ADV. After a median follow-up of 4.5 years, 88 patients developed LRE including 48 with HCC. The cumulative incidence of LRE at year 1, 3, and 5 was 2.1%, 7.0%, and 12.7%, respectively, and was similar for three treatment groups. All models using variables at month 6 or 12 had better fit than models using baseline values. The best model for prediction of LRE used PLT, GGT, and AFP at month 6 [AUC: 0.762 (0.678–0.814)], for hepatic decompensation—PLT, LSM and GGT at month 12 (AUC: 0.834 (0.675–0.919)), and for HCC—AFP and GGT at month 6 [AUC 0.763 (0.691–0.828)]. All models had negative predictive values of 94.0–98.8%.
Conclusion
Models using on-treatment variables are more accurate than models using baseline variables in predicting LRE in patient with compensated HBV-induced cirrhosis receiving antiviral therapy. ClincialTrials.gov number NCT01943617, NCT01720238, NCT03366571, NCT02849132.



Similar content being viewed by others
Abbreviations
- ADV:
-
Adefovir
- ALT:
-
Alanine transaminase
- ALB:
-
Albumin
- AFP:
-
Alpha-fetoprotein
- AST:
-
Aspartate aminotransferase
- CTP:
-
Child-Turcotte-Pugh
- Cr:
-
Creatine
- ETV:
-
Entecavir
- GGT:
-
Gamma-glutamyl transferase
- HCC:
-
Hepatocellular carcinoma
- HE:
-
Hepatoencephalopathy
- INR:
-
International normalized ratio
- LAM:
-
Lamivudine
- LRE:
-
Liver-related event
- LSM:
-
Liver stiffness measurement
- MELD:
-
Model for end-stage liver disease
- PLT:
-
Platelet
- RCT:
-
Randomized controlled trial
- Thy-α1:
-
Thymosin-alpha1
- TB:
-
Total bilirubin
- VB:
-
Variceal bleeding
References
Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–55.
World Health Orgnization. Global hepatitis report-2017. https://www.hoint/hepatitis/publications/global-hepatitis-report2017/en/. 2017. Acessd June 02 2020
van Bommel F, Berg T. Treatment of HBV related cirrhosis. Liver Int. 2013;33(Suppl 1):176–81.
Papatheodoridis GV, Chan HL, Hansen BE, Janssen HL, Lampertico P. Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. J Hepatol. 2015;62(4):956–67.
Tsai MC, Chen CH, Hu TH, Lu SN, Lee CM, Wang JH, Hung CH. Long-term outcomes of hepatitis B virus-related cirrhosis treated with nucleos(t)ide analogs. J Formos Med Assoc. 2017;116(7):512–21.
Yuen MF, Tanaka Y, Fong DY, Fung J, Wong DK, Yuen JC, But DY, Chan AO, Wong BC, Mizokami M, Lai CL. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol. 2009;50(1):80–8.
Wong VW, Chan SL, Mo F, Chan TC, Loong HH, Wong GL, Lui YY, Chan AT, Sung JJ, Yeo W, Chan HL, Mok TS. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol. 2010;28(10):1660–5.
Yang HI, Yuen MF, Chan HLY, Han KH, Chen PJ, Kim DY, Ahn SH, Chen CJ, Wong VWS, Seto WK. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 2011;12(6):568–74.
Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O’Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Inarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–8.
Chen RC, Cai YJ, Wu JM, Wang XD, Song M, Wang YQ, Zheng MH, Chen YP, Lin Z, Shi KQ. Usefulness of albumin-bilirubin grade for evaluation of long-term prognosis for hepatitis B-related cirrhosis. J Viral Hepat. 2017;24(3):238–45.
Jung KS, Kim SU, Song K, Park JY, Kim DY, Ahn SH, Kim BK, Han KH. Validation of hepatitis B virus-related hepatocellular carcinoma prediction models in the era of antiviral therapy. Hepatology. 2015;62(6):1757–66.
Wong GL, Chan HL, Wong CK, Leung C, Chan CY, Ho PP, Chung VC, Chan ZC, Tse YK, Chim AM, Lau TK, Wong VW. Liver stiffness-based optimization of hepatocellular carcinoma risk score in patients with chronic hepatitis B. J Hepatol. 2014;60(2):339–45.
Wu S, Kong Y, Piao H, Jiang W, Xie W, Chen Y, Lu L, Ma A, Xie S, Ding H, Shang J, Zhang X, Feng B, Han T, Xu X, Huo L, Cheng J, Li H, Wu X, Zhou J, Sun Y, Ou X, Zhang H, You H, Jia J. On-treatment changes of liver stiffness at week 26 could predict 2-year clinical outcomes in HBV-related compensated cirrhosis. Liver Int. 2018;38(6):1045–54.
Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, Calleja JL, Chi H, Manolakopoulos S, Mangia G, Gatselis N, Keskin O, Savvidou S, de la Revilla J, Hansen BE, Vlachogiannakos I, Galanis K, Idilman R, Colombo M, Esteban R, Janssen HL, Lampertico P. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64(4):800–6.
Wu X, Shi Y, Zhou J, Sun Y, Piao H, Jiang W, Ma A, Chen Y, Xu M, Xie W, Cheng J, Xie S, Shang J, Cheng J, Xie Q, Ding H, Zhang X, Bai L, Zhang M, Wang B, Chen S, Ma H, Ou X, Jia J, You H. Combination of entecavir with thymosin alpha-1 in HBV-related compensated cirrhosis: a prospective multicenter randomized open-label study. Expert Opin Biol Ther. 2018;18(sup1):61–9.
Wu X, Zhou J, Xie W, Ding H, Ou X, Chen G, Ma A, Xu X, Ma H, Xu Y, Liu X, Meng T, Wang L, Sun Y, Wang B, Kong Y, Ma H, You H, Jia J. Entecavir monotherapy versus de novo combination of lamivudine and adefovir for compensated hepatitis B virus-related cirrhosis: a real-world prospective multicenter cohort study. Infect Drug Resist. 2019;12:745–57.
Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM, Lam AT, Iu HW, Leung JM, Lai JW, Lo AO, Chan HY, Wong VW. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58(5):1537–47.
Goyal SK, Dixit VK, Shukla SK, Ghosh J, Behera M, Tripathi M, Gupta N, Ranjan A, Jain AK. Prolonged use of tenofovir and entecavir in hepatitis B virus-related cirrhosis. Indian J Gastroenterol. 2015;34(4):286–91.
Papatheodoridis GV, Sypsa V, Dalekos G, Yurdaydin C, van Boemmel F, Buti M, Goulis J, Calleja JL, Chi H, Manolakopoulos S, Loglio A, Siakavellas S, Gatselis N, Keskin O, Lehretz M, Savvidou S, de la Revilla J, Hansen BE, Kourikou A, Vlachogiannakos I, Galanis K, Idilman R, Colombo M, Esteban R, Janssen HLA, Berg T, Lampertico P. Eight-year survival in chronic hepatitis B patients under long-term entecavir or tenofovir therapy is similar to the general population. J Hepatol. 2018;68(6):1129–36.
Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–26.
Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, Sulkowski MS, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M. Investigators AC. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–25.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45.
Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351(15):1521–31.
Sheng YJ, Liu JY, Tong SW, Hu HD, Zhang DZ, Hu P, Ren H. Lamivudine plus adefovir combination therapy versus entecavir monotherapy for lamivudine-resistant chronic hepatitis B: a systematic review and meta-analysis. Virol J. 2011;8:393.
Buti M, Riveiro-Barciela M, Esteban R. Long-term safety and efficacy of nucleo(t)side analogue therapy in hepatitis B. Liver Int. 2018;38(Suppl 1):84–9.
Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R, Bornstein JD, Kitrinos KM, Subramanian GM, McHutchison JG, Heathcote EJ. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468–75.
Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z, Chi YC, Zhang H, Hindes R, Iloeje U, Beebe S, Kreter B. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52(3):886–93.
Schiff ER, Lee SS, Chao YC, Kew Yoon S, Bessone F, Wu SS, Kryczka W, Lurie Y, Gadano A, Kitis G, Beebe S, Xu D, Tang H, Iloeje U. Long-term treatment with entecavir induces reversal of advanced fibrosis or cirrhosis in patients with chronic hepatitis B. Clin Gastroenterol Hepatol. 2011;9(3):274–6.
Wang L, Wang B, You H, Wu X, Zhou J, Ou X, Jia J. Platelets’ increase is associated with improvement of liver fibrosis in entecavir-treated chronic hepatitis B patients with significant liver fibrosis. Hepatol Int. 2018;12(3):237–43.
Chon YE, Park JY, Myoung SM, Jung KS, Kim BK, Kim SU, Kim DY, Ahn SH, Han KH. Improvement of liver fibrosis after long-term antiviral therapy assessed by fibroscan in chronic hepatitis B patients with advanced fibrosis. Am J Gastroenterol. 2017;112(6):882–91.
Wang J, Zhang Z, Yan X, Li M, Xia J, Liu Y, Chen Y, Jia B, Zhu L, Zhu C, Huang R, Wu C. Albumin-bilirubin (ALBI) as an accurate and simple prognostic score for chronic hepatitis B-related liver cirrhosis. Dig Liver Dis. 2019;51(8):1172–8.
Acknowledgements
I would like to give special thanks to Dr. Anna Suk-Fong, Lok for her numerous advice on data analysis and review of the manuscript. I am grateful for your instruction on being a good doctor, for your guidance me on becoming an excellent clinical researcher, for your warm support through tough times, and for your strong encouragement. It is an unbelievable and precious experience in my life to have had the opportunity to work with you this past year.
Funding
This study was funded by the National Major Science and Technology Project (2018ZX10302204-004, 2018ZX09201016, 2017ZX10203202-003), the Project of Beijing Municipal Commission of Science and Technology (D161100002716003), and the Project of the Digestive Medical Coordinated Development Center of Beijing Hospitals Authority (XXZ0405).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Xiaoning Wu, Jialing Zhou, Yameng Sun, Huiguo Ding, Guofeng Chen, Wen Xie,Hongxin Piao, Xiaoyuan Xu, Wei Jiang, Hui Ma, Anlin Ma, Yongpeng Chen, Mingyi Xu, Jilin Cheng, Youqing Xu, Tongtong Meng, Bingqiong Wang, Shuyan Chen, Yiwen Shi, Yuanyuan Kong, Xiaojuan Ou, Hong You, Jidong Jia authors have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The protocol, patient information sheets, and consent forms were approved by the Medical Ethics Committee of Beijing Friendship Hospital, Capital Medical University (BJFH-EC/2013-029, BJFH-EC/2013-067, 2016-P2-021-01, 2016-P2-022-01) and the other 33 participating centers. The studies were registered with the ClinicalTrials.gov identifier NCT01943617, NCT01720238, NCT03366571, NCT02849132.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12072_2020_10114_MOESM1_ESM.tif
Supplementary file1 Supplementary figure 1. Cumulative incidence of LRE, decompensation and HCC had no statistical differences among three treatment groups. LRE, liver related events; HCC, hepatocellular carcinoma (TIF 58 kb)
12072_2020_10114_MOESM2_ESM.tif
Supplementary file1 Supplementary figure 2. Long-term virological response and biochemical response in patients with and without LRE. A. HBV DNA decreased rapidly after initiation of antiviral treatment and maintained. B. More than 90% of patients achieved HBV DNA< 200 IU/mL after 1 year antiviral treatment and maintained. C, E,F. AST, ALB, GGT improved rapidly during the first 6 months (all P <0.01), and then continued to improve slowly but significantly to 2 years in LRE group and to 3 years in non-LRE group, and remained stable thereafter. D. TB had no significant change because most patients were within normal range. AST, alanine aminotransferase; TB, total bilirubin; ALB, albumin; GGT, Gamma-glutamyl transferase (TIF 117 kb)
12072_2020_10114_MOESM3_ESM.tif
Supplementary file1 Supplementary figure 3. On-treatment change of PLT, AFP and liver cirrhosis related indices. PLT increased significantly from baseline to year 5 in both LRE group (median 78 to 124 ×109/L, P<0.01) and non-LRE group (median 96 to 140 ×109/L, P<0.01), but the increase was slower in LRE group and stabilized after 2 years, while progressive increase was observed in non-LRE group. AFP in LRE group decreased from baseline to year 2 (P < 0.001) and then kept stable, it continually improved from baseline to year 5 significantly in non-LRE group. LSM improved rapidly during the first 6 months (all P <0.01), and then continued to improve slowly but significantly to 2 years in LRE group and to 3 years in non-LRE group, and remained stable thereafter. INR in LRE group decreased during the first year and then increased, it continually improved from baseline to year 5 significantly in non-LRE group. APRI and FIB-4 decreased rapidly during 6 months and decreased slowly to year 2 and maintained. LRE, liver related event; PLT, platelet; AFP, alpha-fetoprotein; LSM, liver stiffness measurement; INR, international normalized ratio; APRI, AST to PLT ratio index; FIB-4, fibrosis-4 (TIF 101 kb)
12072_2020_10114_MOESM4_ESM.tif
Supplementary file1 Supplementary figure 4. On-treatment changes of CTP, MELD ALBI. CTP and MELD showed no significant change after initiation of antiviral therapy. ALBI decreased gradually as albumin increased. CTP, Child-Turcotte-Pugh; MELD, model for end-stage liver disease; ALBI, albumin-bilirubin(TIF 48 kb)
Rights and permissions
About this article
Cite this article
Wu, X., Zhou, J., Sun, Y. et al. Prediction of liver-related events in patients with compensated HBV-induced cirrhosis receiving antiviral therapy. Hepatol Int 15, 82–92 (2021). https://doi.org/10.1007/s12072-020-10114-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-020-10114-1