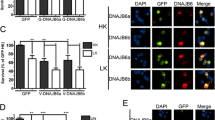
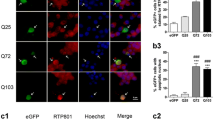
Abstract
Proteins belonging to the AP-1 family of transcription factors are known to be involved in the regulation of neuronal viability. While strides have been made to elucidate the mechanisms of how individual members regulate cell death, much remains unknown. We find that the expression of one AP-1 member, c-Fos, is reduced in cerebellar granule neurons (CGNs) induced to die by low potassium (LK) treatment. Restoration and increase of this expression protect CGNs against LK-induced death, whereas knockdown induces death of otherwise healthy neurons. Furthermore, forced expression can protect cortical neurons against homocysteic acid (HCA)-induced toxicity. Taken together, this suggests that c-Fos is necessary for neuronal survival and that elevating c-Fos expression has a neuroprotective effect. Consistent with this idea is the finding that c-Fos expression is reduced selectively in the striatum in two separate mouse models of Huntington’s disease and forced expression protects against neuronal death resulting from mutant huntingtin (mut-Htt) expression. Interestingly, neuroprotection by c-Fos does not require its DNA-binding, transcriptional, or heteromerization domains. However, this protective activity can be inhibited by pharmacological inhibition of c-Abl, CK-I, and MEK-ERK signaling. Additionally, expression of point mutant forms of this protein has identified that mutation of a tyrosine residue, Tyr345, can convert c-Fos from neuroprotective to neurotoxic. We show that c-Fos interacts with histone deacetylase-3 (HDAC3), a protein that contributes to mut-Htt neurotoxicity and whose overexpression is sufficient to promote neuronal death. When co-expressed, c-Fos can protect against HDAC3 neurotoxicity. Finally, our study identifies a 21-amino acid region at the C-terminus of c-Fos that is sufficient to protect neurons against death induced by LK, HCA treatment, or mut-Htt expression when expressed via a plasmid transfection or as a cell-permeable peptide. This cell-permeable peptide, designated as Fos-CTF, could have potential as a therapeutic agent for neurodegenerative diseases.











Similar content being viewed by others
References
Angel P, Szabowski A, Schorpp-Kistner M (2001) Function and regulation of AP-1 subunits in skin physiology and pathology. Oncogene 20:2413–2423
Chinenov Y, Kerppola TK (2001) Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 20:2438–2452
Jochum W, Passegue E, Wagner EF (2001) AP-1 in mouse development and tumorigenesis. Oncogene 20:2401–2412
Shaulian E, Karin M (2001) AP-1 in cell proliferation and survival. Oncogene 20:2390–2400
D’Alonzo RC, Selvamurugan N, Karsenty G, Partridge NC (2002) Physical interaction of the activator protein-1 factors c-Fos and c-Jun with Cbfa1 for collagenase-3 promoter activation. J Biol Chem 277:816–822
Gao Z, Ye J (2008) Inhibition of transcriptional activity of c-JUN by SIRT1. Biochem Biophys Res Commun 376:793–796
Ivorra C, Kubicek M, Gonzalez JM, Sanz-Gonzalez SM, Alvarez-Barrientos A, O’Connor JE, Burke B, Andres V (2006) A mechanism of AP-1 suppression through interaction of c-Fos with lamin A/C. Genes Dev 20:307–320
Liang CL, Chen JL, Hsu YP, Ou JT, Chang YS (2002) Epstein-Barr virus BZLF1 gene is activated by transforming growth factor-beta through cooperativity of Smads and c-Jun/c-Fos proteins. J Biol Chem 277:23345–23357
Zhang Y, Zhao Y, Li H, Li Y, Cai X, Shen Y, Shi H, Li L, Liu Q, Zhang X, Ye L (2013) The nuclear import of oncoprotein hepatitis B X-interacting protein depends on interacting with c-Fos and phosphorylation of both proteins in breast cancer cells. J Biol Chem 288:18961–18974
Ham J, Babij C, Whitfield J, Pfarr CM, Lallemand D, Yaniv M, Rubin LL (1995) A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron 14:927–939
Estus S, Zaks WJ, Freeman RS, Gruda M, Bravo R, Johnson EM Jr (1994) Altered gene expression in neurons during programmed cell death: identification of c-Jun as necessary for neuronal apoptosis. J Cell Biol 127:1717–1727
Schenkel J (2004) Activation of the c-Jun transcription factor following neurodegeneration in vivo. Neurosci Lett 361:36–39
Yuan Z, Gong S, Luo J, Zheng Z, Song B, Ma S, Guo J, Hu C, Thiel G, Vinson C, Hu CD, Wang Y, Li M (2009) Opposing roles for ATF2 and c-Fos in c-Jun-mediated neuronal apoptosis. Mol Cell Biol 29:2431–2442
Bjorkblom B, Vainio JC, Hongisto V, Herdegen T, Courtney MJ, Coffey ET (2008) All JNKs can kill, but nuclear localization is critical for neuronal death. J Biol Chem 283:19704–19713
Chen HM, Wang L, D’Mello SR (2008) Inhibition of ATF-3 expression by B-Raf mediates the neuroprotective action of GW5074. J Neurochem 105:1300–1312
Zhang J, Zhang D, McQuade JS, Behbehani M, Tsien JZ, Xu M (2002) C-Fos regulates neuronal excitability and survival. Nat Genet 30:416–420
Wacker JL, Huang SY, Steele AD, Aron R, Lotz GP, Nguyen Q, Giorgini F, Roberson ED, Lindquist S, Masliah E, Muchowski PJ (2009) Loss of Hsp70 exacerbates pathogenesis but not levels of fibrillar aggregates in a mouse model of Huntington’s disease. J Neurosci 29:9104–9114
Anglada-Huguet M, Giralt A, Perez-Navarro E, Alberch J, Xifro X (2012) Activation of Elk-1 participates as a neuroprotective compensatory mechanism in models of Huntington’s disease. J Neurochem 121:639–648
Ferrara P, Andermarcher E, Bossis G, Acquaviva C, Brockly F, Jariel-Encontre I, Piechaczyk M (2003) The structural determinants responsible for c-Fos protein proteasomal degradation differ according to the conditions of expression. Oncogene 22:1461–1474
Bossis G, Ferrara P, Acquaviva C, Jariel-Encontre I, Piechaczyk M (2003) c-Fos proto-oncoprotein is degraded by the proteasome independently of its own ubiquitinylation in vivo. Mol Cell Biol 23:7425–7436
D’Mello SR, Galli C, Ciotti T, Calissano P (1993) Induction of apoptosis in cerebellar granule neurons by low potassium: inhibition of death by insulin-like growth factor I and cAMP. Proc Natl Acad Sci U S A 90:10989–10993
D’Mello SR, Borodezt K, Soltoff SP (1997) Insulin-like growth factor and potassium depolarization maintain neuronal survival by distinct pathways: possible involvement of PI 3-kinase in IGF-1 signaling. J Neurosci 17:1548–1560
Yalcin A, Koulich E, Mohamed S, Liu L, D’Mello SR (2003) Apoptosis in cerebellar granule neurons is associated with reduced interaction between CREB-binding protein and NF-kappaB. J Neurochem 84:397–408
Dastidar SG, Landrieu PM, D’Mello SR (2011) FoxG1 promotes the survival of postmitotic neurons. J Neurosci 31:402–413
Majdzadeh N, Wang L, Morrison BE, Bassel-Duby R, Olson EN, D’Mello SR (2008) HDAC4 inhibits cell-cycle progression and protects neurons from cell death. Dev Neurobiol 68:1076–1092
Bardai FH, Verma P, Smith C, Rawat V, Wang L, D’Mello SR (2013) Disassociation of histone deacetylase-3 from normal huntingtin underlies mutant huntingtin neurotoxicity. J Neurosci 33:11833–11838
Bardai FH, Price V, Zaayman M, Wang L, D’Mello SR (2012) Histone deacetylase-1 (HDAC1) is a molecular switch between neuronal survival and death. J Biol Chem 287:35444–35453
Wang L, Ankati H, Akubathini SK, Balderamos M, Storey CA, Patel AV, Price V, Kretzschmar D, Biehl ER, D’Mello SR (2010) Identification of novel 1,4-benzoxazine compounds that are protective in tissue culture and in vivo models of neurodegeneration. J Neurosci Res 88:1970–1984
Chin PC, Liu L, Morrison BE, Siddiq A, Ratan RR, Bottiglieri T, D’Mello SR (2004) The c-Raf inhibitor GW5074 provides neuroprotection in vitro and in an animal model of neurodegeneration through a MEK-ERK and Akt-independent mechanism. J Neurochem 90:595–608
Galli C, Meucci O, Scorziello A, Werge TM, Calissano P, Schettini G (1995) Apoptosis in cerebellar granule cells is blocked by high KCl, forskolin, and IGF-1 through distinct mechanisms of action: the involvement of intracellular calcium and RNA synthesis. J Neurosci 15:1172–1179
Ratan RR, Murphy TH, Baraban JM (1994) Oxidative stress induces apoptosis in embryonic cortical neurons. J Neurochem 62:376–379
Ratan RR, Murphy TH, Baraban JM (1994) Macromolecular synthesis inhibitors prevent oxidative stress-induced apoptosis in embryonic cortical neurons by shunting cysteine from protein synthesis to glutathione. J Neurosci 14:4385–4392
Chen RH, Abate C, Blenis J (1993) Phosphorylation of the c-Fos transrepression domain by mitogen-activated protein kinase and 90-kDa ribosomal S6 kinase. Proc Natl Acad Sci U S A 90:10952–10956
Okazaki K, Sagata N (1995) The Mos/MAP kinase pathway stabilizes c-Fos by phosphorylation and augments its transforming activity in NIH 3T3 cells. EMBO J 14:5048–5059
Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J (2002) Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol 4:556–564
Schuermann M, Neuberg M, Hunter JB, Jenuwein T, Ryseck RP, Bravo R, Muller R (1989) The leucine repeat motif in Fos protein mediates complex formation with Jun/AP-1 and is required for transformation. Cell 56:507–516
Neuberg M, Adamkiewicz J, Hunter JB, Muller R (1989) A Fos protein containing the Jun leucine zipper forms a homodimer which binds to the AP1 binding site. Nature 341:243–245
Cohen DR, Curran T (1990) Analysis of dimerization and DNA binding functions in Fos and Jun by domain-swapping: involvement of residues outside the leucine zipper/basic region. Oncogene 5:929–939
Borlongan CV, Koutouzis TK, Sanberg PR (1997) 3-Nitropropionic acid animal model and Huntington’s disease. Neurosci Biobehav Rev 21:289–293
Brouillet E, Jacquard C, Bizat N, Blum D (2005) 3-Nitropropionic acid: a mitochondrial toxin to uncover physiopathological mechanisms underlying striatal degeneration in Huntington’s disease. J Neurochem 95:1521–1540
Wang L, Ankati H, Akubathini SK, Balderamos M, Storey CA, Patel AV, Price V, Kretzschmar D, Biehl ER, D’Mello SR (2010) Identification of novel 1,4-benzoxazine compounds that are protective in tissue culture and in vivo models of neurodegeneration. J Neurosci Res 88:1970–1984
Jia H, Pallos J, Jacques V, Lau A, Tang B, Cooper A, Syed A, Purcell J, Chen Y, Sharma S, Sangrey GR, Darnell SB, Plasterer H, Sadri-Vakili G, Gottesfeld JM, Thompson LM, Rusche JR, Marsh JL, Thomas EA (2012) Histone deacetylase (HDAC) inhibitors targeting HDAC3 and HDAC1 ameliorate polyglutamine-elicited phenotypes in model systems of Huntington’s disease. Neurobiol Dis 46:351–361
Thomas EA, Coppola G, Desplats PA, Tang B, Soragni E, Burnett R, Gao F, Fitzgerald KM, Borok JF, Herman D, Geschwind DH, Gottesfeld JM (2008) The HDAC inhibitor 4b ameliorates the disease phenotype and transcriptional abnormalities in Huntington’s disease transgenic mice. Proc Natl Acad Sci U S A 105:15564–15569
Steffan JS, Bodai L, Pallos J, Poelman M, McCampbell A, Apostol BL, Kazantsev A, Schmidt E, Hu YZ, Greenwald M, Kurokawa R, Housman DE, Jackson GR, Marsh JL, Thompson LM (2001) Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 413:739–43
Pallos J, Bodai L, Lukacsovich T, Purcell JM, Steffan JS, Thompson LM, Marsh JL (2008) Inhibition of specific HDACs and sirtuins suppresses pathogenesis in a Drosophila model of Huntington’s disease. Hum Mol Genet 17:3767–3775
Ferrante RJ, Kubilus JK, Lee J, Ryu H, Beesen A, Zucker B, Smith K, Kowall NW, Ratan RR, Luthi-Carter R, Hersch SM (2003) Histone deacetylase inhibition by sodium butyrate chemotherapy ameliorates the neurodegenerative phenotype in Huntington’s disease mice. J Neurosci 23:9418–9427
Hockly E, Richon VM, Woodman B, Smith DL, Zhou X, Rosa E, Sathasivam K, Ghazi-Noori S, Mahal A, Lowden PA, Steffan JS, Marsh JL, Thompson LM, Lewis CM, Marks PA, Bates GP (2003) Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington’s disease. Proc Natl Acad Sci U S A 100:2041–2046
Bardai FH, D’Mello SR (2011) Selective toxicity by HDAC3 in neurons: regulation by Akt and GSK3beta. J Neurosci 31:1746–1751
Zhao K, Ippolito G, Wang L, Price V, Kim MH, Cornwell G, Fulenchek S, Breen GA, Goux WJ, D’Mello SR (2010) Neuron-selective toxicity of tau peptide in a cell culture model of neurodegenerative tauopathy: essential role for aggregation in neurotoxicity. J Neurosci Res 88:3399–3413
Zaidi N, Burster T, Sommandas V, Herrmann T, Boehm BO, Driessen C, Voelter W, Kalbacher H (2007) A novel cell penetrating aspartic protease inhibitor blocks processing and presentation of tetanus toxoid more efficiently than pepstatin A. Biochem Biophys Res Commun 364:243–249
Zhai D, Luciano F, Zhu X, Guo B, Satterthwait AC, Reed JC (2005) Humanin binds and nullifies Bid activity by blocking its activation of Bax and Bak. J Biol Chem 280:15815–15824
D’Mello SR (2009) Histone deacetylases as targets for the treatment of human neurodegenerative diseases. Drug News Perspect 22:513–524
Kazantsev AG, Thompson LM (2008) Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat Rev Drug Discov 7:854–868
Sleiman SF, Basso M, Mahishi L, Kozikowski AP, Donohoe ME, Langley B, Ratan RR (2009) Putting the ‘HAT’ back on survival signalling: the promises and challenges of HDAC inhibition in the treatment of neurological conditions. Expert Opin Investig Drugs 18:573–584
Acknowledgments
This work was supported by NIH grant R01 NS040408 to SRD. We thank Eric J. Nestler of the Mount Sinai Medical Center, NY for the FosB and ΔFosB plasmids, Troy Littleton of MIT for the wild-type and mutant Htt plasmids, and Rajiv R. Ratan of the Burke Institute of Medical Research, NY, for the HT22 cell line, Jason A. Pfister, Jade M. Franklin and Chad C. Smith for reading the manuscript and providing comments.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Rawat, V., Goux, W., Piechaczyk, M. et al. c-Fos Protects Neurons Through a Noncanonical Mechanism Involving HDAC3 Interaction: Identification of a 21-Amino Acid Fragment with Neuroprotective Activity. Mol Neurobiol 53, 1165–1180 (2016). https://doi.org/10.1007/s12035-014-9058-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-9058-1