Abstract
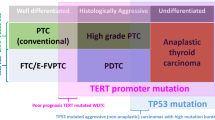
Poorly differentiated thyroid carcinoma (PDTC) and anaplastic thyroid carcinoma (ATC) are aggressive thyroid tumors associated with a high mortality rate of 38–57 % and almost 100 % respectively. Several recent studies utilizing next generation sequencing techniques have shed lights on the molecular pathogenesis of these tumors, providing evidence to support a stepwise tumoral progression from well-differentiated to poorly differentiated, and finally to anaplastic thyroid carcinomas. While BRAF V600E and RAS mutations remain the main drivers in aggressive thyroid carcinoma, PDTC and ATC gains additional mutations, e.g., TERT promoter mutation, TP53 mutation, as well as frequent alterations in PIK3CA-PTEN-AKT-mTOR pathway, SWI-SNF complex, histomethyltransferases, and mismatch repair genes. RAS-mutated PDTCs are commonly associated with a histologic phenotype defined by Turin proposal, high frequency of distant metastasis, high thyroid differentiation score, and a RAS-like gene expression profile, whereas BRAF-mutated PDTCs are usually defined solely by the Memorial Sloan Kettering Cancer Center (MSKCC) criteria with a propensity for nodal metastasis and are less differentiated with a BRAF-like expression signature. Such demarcation is largely lost in ATC which is characterized by genomic complexity, heavy mutation burden, and profound undifferentiation. Additionally, several molecular events, e.g., EIF1AX mutation, mutation burden, and chromosome 1q gain in PDTCs, as well as EIF1AX mutation, chromosome 13q loss, and 20q gains in ATCs, may serve as adverse prognostic markers predicting poor clinical outcome.




Similar content being viewed by others
References
Siegel R, Ma J, Zou Z, Jemal A Cancer statistics, 2014. CA: a cancer journal for clinicians 64: 9–29, 2014.
Delellis RA, Lloyd RV, Heitz RU, Eng C: Pathology and Genetics of Tumours of Endocrine Organs, Lyon, France: International Agency for Research on Cancer (IARC) Press, 2004.
Hiltzik D, Carlson DL, Tuttle RM et al. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer 106: 1286–1295, 2006.
Volante M, Collini P, Nikiforov YE et al. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. The American journal of surgical pathology 31: 1256–1264, 2007.
Integrated genomic characterization of papillary thyroid carcinoma. Cell 159: 676–690, 2014.
Landa I, Ibrahimpasic T, Boucai L et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. The Journal of clinical investigation 126: 1052–1066, 2016.
Jeon M, Chun SM, Kim D et al. Genomic alterations of anaplastic thyroid carcinoma detected by targeted massive parallel sequencing in a BRAFV600E mutation-prevalent area. Thyroid: official journal of the American Thyroid Association, 2016.
Sykorova V, Dvorakova S, Vcelak J et al. Search for new genetic biomarkers in poorly differentiated and anaplastic thyroid carcinomas using next generation sequencing. Anticancer research 35: 2029–2036, 2015.
Kunstman JW, Juhlin CC, Goh G et al. Characterization of the mutational landscape of anaplastic thyroid cancer via whole-exome sequencing. Hum Mol Genet 24: 2318–2329, 2015.
Carcangiu ML, Zampi G, Rosai J Poorly differentiated (“insular”) thyroid carcinoma. A reinterpretation of Langhans’ “wuchernde Struma”. The American journal of surgical pathology 8: 655–668, 1984.
Xu B, Ibrahimpasic T, Wang L, Tuttle RM, Ganly I, Ghossein R Clinico-pathologic features of fatal non-anaplastic follicular cell-derived thyroid carcinomas (non-ANA FCDCs). Mod Pathol 29(S2): 157, 2016.
Rivera M, Ghossein RA, Schoder H, Gomez D, Larson SM, Tuttle RM Histopathologic characterization of radioactive iodine-refractory fluorodeoxyglucose-positron emission tomography-positive thyroid carcinoma. Cancer 113: 48–56, 2008.
Caillou B, Talbot M, Weyemi U et al. Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma. PloS one 6: e22567, 2011
Ryder M, Ghossein RA, Ricarte-Filho JC, Knauf JA, Fagin JA Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocrine-related cancer 15: 1069–1074, 2008.
Cheng DT, Mitchell TN, Zehir A et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. The Journal of molecular diagnostics: JMD 17: 251–264, 2015.
Nikiforova MN, Lynch RA, Biddinger PW et al. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: evidence for distinct molecular pathways in thyroid follicular carcinoma. The Journal of clinical endocrinology and metabolism 88: 2318–2326, 2003.
Akincilar SC, Unal B, Tergaonkar V Reactivation of telomerase in cancer. Cellular and molecular life sciences : CMLS 73: 1659–1670, 2016.
Melo M, da Rocha AG, Vinagre J et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. The Journal of clinical endocrinology and metabolism 99: E754–E765, 2014
De-Tao Y, Kun Y, Run-Qing L et al. Clinicopathological significance of TERT promoter mutation in papillary thyroid carcinomas: a systematic review and meta-analysis. Clin Endocrinol (Oxf), 2016.
Landa I, Ganly I, Chan TA et al. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. The Journal of clinical endocrinology and metabolism 98: E1562–E1566, 2013
Liu X, Bishop J, Shan Y et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocrine-related cancer 20: 603–610, 2013.
Song YS, Lim JA, Choi H et al. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer, 2016.
Karunamurthy A, Panebianco F, Hsiao S et al. Prevalence and phenotypic characteristics of EIF1AX mutations in thyroid nodules. Endocrine-related cancer, 2016.
Fagin JA, Matsuo K, Karmakar A, Chen DL, Tang SH, Koeffler HP High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. The Journal of clinical investigation 91: 179–184, 1993
Ito T, Seyama T, Mizuno T et al. Unique association of p53 mutations with undifferentiated but not with differentiated carcinomas of the thyroid gland. Cancer Res 52: 1369–1371, 1992.
Gao J, Aksoy BA, Dogrusoz U et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Science signaling 6: pl1, 2013.
Garcia-Rostan G, Camp RL, Herrero A, Carcangiu ML, Rimm DL, Tallini G Beta-catenin dysregulation in thyroid neoplasms: down-regulation, aberrant nuclear expression, and CTNNB1 exon 3 mutations are markers for aggressive tumor phenotypes and poor prognosis. Am J Pathol 158: 987–996, 2001
Garcia-Rostan G, Tallini G, Herrero A, D'Aquila TG, Carcangiu ML, Rimm DL Frequent mutation and nuclear localization of beta-catenin in anaplastic thyroid carcinoma. Cancer Res 59: 1811–1815, 1999
Kurihara T, Ikeda S, Ishizaki Y et al. Immunohistochemical and sequencing analyses of the Wnt signaling components in Japanese anaplastic thyroid cancers. Thyroid: official journal of the American Thyroid Association 14: 1020–1029, 2004.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Xu, B., Ghossein, R. Genomic Landscape of poorly Differentiated and Anaplastic Thyroid Carcinoma. Endocr Pathol 27, 205–212 (2016). https://doi.org/10.1007/s12022-016-9445-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-016-9445-4