Abstract
Purpose
Non-functioning pituitary adenoma (NFPA) is the most prevalent pituitary macroadenoma. No prognostic marker has been found to explain the behavior of these tumors. We aimed to explore cell proliferation, apoptosis, proangiogenic markers, and microvascular density (MVD) in noninvasive and invasive NFPAs.
Methods
Adenoma invasiveness was defined according to Knosp and Hardy classifications based on preoperative magnetic resonance imaging scans. Cell proliferation was examined using Ki67 and P53. Tissue expression of Bcl-2 was used to assess the antiapoptosis pathway. CD34 and CD105 were measured to evaluate MVD, while VEGF expression was assessed as an indicator of pro-angiogenesis. Moreover, VEGF, bFGF, endocan, and endostatin were measured on preoperative serum samples.
Results
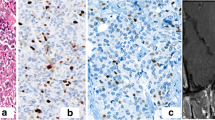
Tissue and serum markers were examined in 18 patients with invasive and 21 patients with noninvasive NFPAs. Ki67 less than 3% was reported in 10 invasive and 14 noninvasive NFPAs (P = 0.752). P53 staining was negative in all subjects. In addition, Bcl-2 staining was negative in 15 and 20 subjects, respectively (P = 0.718). VEGF-A expression 2+ or 3+ was reported in 9 invasive and 11 noninvasive macroadenomas (P = 0.83). Moreover, CD34 and CD105 positivity were comparable between the two groups. Furthermore, the comparison of serum markers showed no significant differences.
Conclusion
Cell proliferation, apoptosis, and angiogenesis play a limited role in NFPA behavior.

Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
M. Mercado, V. Melgar, L. Salame, D. Cuenca, Clinically non-functioning pituitary adenomas: pathogenic, diagnostic and therapeutic aspects. Endocrinologia, diabetes y nutricion 64(7), 384–395 (2017)
M.M. Fernández-Balsells, M.H. Murad, A. Barwise, J.F. Gallegos-Orozco, A. Paul, M.A. Lane, J.F. Lampropulos, I. Natividad, L. Perestelo-Perez, P.G. Ponce de Leon-Lovaton, Natural history of nonfunctioning pituitary adenomas and incidentalomas: a systematic review and metaanalysis. J. Clin. Endocrinol. Metabol. 96(4), 905–912 (2011)
Y. Miao, M. Zong, T. Jiang, X. Yuan, S. Guan, Y. Wang, D. Zhou, A comparative analysis of ESM-1 and vascular endothelial cell marker (CD34/CD105) expression on pituitary adenoma invasion. Pituitary 19(2), 194–201 (2016)
M.E. Molitch, Diagnosis and treatment of pituitary adenomas: a review. JAMA 317(5), 516–524 (2017)
G. Ntali, J.A. Wass, Epidemiology, clinical presentation and diagnosis of non-functioning pituitary adenomas. Pituitary 21(2), 111–118 (2018)
A. Fernandez, N. Karavitaki, J.A. Wass, Prevalence of pituitary adenomas: a community‐based, cross‐sectional study in Banbury (Oxfordshire, UK). Clin. Endocrinol. 72(3), 377–382 (2010)
M. Sato, R. Tamura, H. Tamura, T. Mase, K. Kosugi, Y. Morimoto, K. Yoshida, M. Toda, Analysis of tumor angiogenesis and immune microenvironment in non-functional pituitary endocrine tumors. J. Clin. Med. 8(5), 695 (2019)
H. Turner, Z. Nagy, K. Gatter, M. Esiri, J. Wass, A. Harris, Proliferation, bcl-2 expression and angiogenesis in pituitary adenomas: relationship to tumour behaviour. Br. J. Cancer 82(8), 1441–1445 (2000)
T.-W. Noh, H.J. Jeong, M.-K. Lee, T.S. Kim, S.H. Kim, E.J. Lee, Predicting recurrence of nonfunctioning pituitary adenomas. J. Clin. Endocrinol. Metab. 94(11), 4406–4413 (2009)
A. Di Ieva, A. Weckman, J. Di Michele, F. Rotondo, F. Grizzi, K. Kovacs, M.D. Cusimano, Microvascular morphometrics of the hypophysis and pituitary tumors: from bench to operating theatre. Microvasc. Res. 89, 7–14 (2013)
C. Cristina, G.M. Luque, G. Demarchi, F.L. Vicchi, L. Zubeldia-Brenner, M.I.P. Millan, S. Perrone, A.M. Ornstein, I.M. Lacau-Mengido, S.I. Berner, D. Becu-Villalobos, Angiogenesis in pituitary adenomas: human studies and new mutant mouse models. Int. J. Endocrinol. 2014, 1–11 (2014)
M. Niveiro, F.I. Aranda, G. Peiró, C. Alenda, A. Picó, Immunohistochemical analysis of tumor angiogenic factors in human pituitary adenomas. Hum. Pathol. 36(10), 1090–1095 (2005)
A. Cornelius, C. Cortet‐Rudelli, R. Assaker, O. Kerdraon, M.H. Gevaert, V. Prévot, P. Lassalle, J. Trouillas, M. Delehedde, C.A. Maurage, Endothelial expression of endocan is strongly associated with tumor progression in pituitary adenoma. Brain Pathol. 22(6), 757–764 (2012)
C. Cristina, M.I. Perez-Millan, G. Luque, R.A. Dulce, G. Sevlever, S.I. Berner, D. Becu-Villalobos, VEGF and CD31 association in pituitary adenomas. Endocr. Pathol. 21(3), 154–160 (2010)
K.-M. Lee, S.-H. Park, K.-S. Park, J.-H. Hwang, S.-K. Hwang, Analysis of circulating endostatin and vascular endothelial growth factor in patients with pituitary adenoma treated by stereotactic radiosurgery: a preliminary study. Brain Tumor Res. Treat. 3(2), 89–94 (2015)
F. Grimm, R. Maurus, R. Beschorner, G. Naros, M. Stanojevic, I. Gugel, S. Giese, G. Bier, B. Bender, J. Honegger, Ki-67 labeling index and expression of p53 are non-predictive for invasiveness and tumor size in functional and nonfunctional pituitary adenomas. Acta Neurochirurgica 161(6), 1149–1156 (2019)
O. Mete, M.B. Lopes, Overview of the 2017 WHO classification of pituitary tumors. Endocr. Pathol. 28(3), 228–243 (2017)
N. Weidner, J.P. Semple, W.R. Welch, J. Folkman, Tumor angiogenesis and metastasis—correlation in invasive breast carcinoma. New Engl. J. Med. 324(1), 1–8 (1991)
I.-M. Moldovan, S. Şuşman, R. Pirlog, E.M. Jianu, D.C. Leucuţa, C.S. Melincovici, D. Crişan, I.Ş. Florian, Molecular markers in the diagnosis of invasive pituitary adenomas–an immunohistochemistry study. Rom. J. Morphol. Embryol. 58(4), 1357–1364 (2017)
E. Knosp, E. Steiner, K. Kitz, C. Matula, Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 33(4), 610–618 (1993)
J. Hardy, Transphenoidal microsurgical treatment of pituitary. In: J.A. Linfoot (ed.) Recent advances in the diagnosis and treatment of pituitary tumors. (Raven press, New York, 1979), pp. 375–378
K. Kovacs, Tumors of the pituitary gland. Atlas Tumor Pathol Fascicle 21, 1–269 (1986). 2nd series
B.M. Davies, E. Carr, C. Soh, K.K. Gnanalingham, Assessing size of pituitary adenomas: a comparison of qualitative and quantitative methods on MR. Acta Neurochirurgica 158(4), 677–683 (2016)
G. Raverot, P. Burman, A. McCormack, A. Heaney, S. Petersenn, V. Popovic, J. Trouillas, O.M. Dekkers, European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur. J. Endocrinol. 178(1), G1–G24 (2018)
J. Trouillas, P. Roy, N. Sturm, E. Dantony, C. Cortet-Rudelli, G. Viennet, J.-F. Bonneville, R. Assaker, C. Auger, T. Brue, A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case–control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. 126(1), 123–135 (2013)
Glebauskiene, B., Liutkeviciene, R., Vilkeviciute, A., Gudinaviciene, I., Rocyte, A., Simonaviciute, D., Mazetyte, R., Kriauciuniene, L., Zaliuniene, D. Association of Ki-67 labelling index and IL-17A with pituitary adenoma. BioMed Res. Int. 2018, https://doi.org/10.1155/2018/7490585 (2018)
R. Hasanov, B.İ. Aydoğan, S. Kiremitçi, E. Erden, S. Güllü, The prognostic roles of the Ki-67 proliferation index, P53 expression, mitotic index, and radiological tumor invasion in pituitary adenomas. Endocr. Pathol. 30(1), 49–55 (2019)
K. Thapar, K. Kovacs, B.W. Scheithauer, L. Stefaneanu, E. Horvath, J. Peter, P., D. Murray, E.R. Laws Jr, Proliferative activity and invasiveness among pituitary adenomas and carcinomas: an analysis using the MIB-1 antibody. Neurosurgery 38(1), 99–107 (1996)
L. Mastronardi, A. Guiducci, C. Spera, F. Puzzilli, F. Liberati, G. Maira, Ki-67 labelling index and invasiveness among anterior pituitary adenomas: analysis of 103 cases using the MIB-1 monoclonal antibody. J. Clin. Pathol. 52(2), 107–111 (1999)
A.M. Landolt, T. Shibata, P. Kleihues, Growth rate of human pituitary adenomas. J. Neurosurg. 67(6), 803–806 (1987)
C.P. Miermeister, S. Petersenn, M. Buchfelder, R. Fahlbusch, D.K. Lüdecke, A. Hölsken, M. Bergmann, H.U. Knappe, V.H. Hans, J. Flitsch, Histological criteria for atypical pituitary adenomas–data from the German pituitary adenoma registry suggests modifications. Acta Neuropathologica Commun. 3(1), 50 (2015)
R. Gejman, B. Swearingen, E.T. Hedley-Whyte, Role of Ki-67 proliferation index and p53 expression in predicting progression of pituitary adenomas. Hum. Pathol. 39(5), 758–766 (2008)
S. Ding, C. Li, S. Lin, Y. Yang, D. Liu, Y. Han, Y. Zhang, L. Li, L. Zhou, S. Kumar, Comparative evaluation of microvessel density determined by CD34 or CD105 in benign and malignant gastric lesions. Hum. Pathol. 37(7), 861–866 (2006)
F. Rotondo, S. Sharma, B. Scheithauer, E. Horvath, L. Syro, M. Cusimano, F. Nassiri, G. Yousef, K. Kovacs, Endoglin and CD-34 immunoreactivity in the assessment of microvessel density in normal pituitary and adenoma subtypes. Neoplasma 57(6), 590 (2010)
S. Vidal, K. Kovacs, E. Horvath, B.W. Scheithauer, T. Kuroki, R.V. Lloyd, Microvessel density in pituitary adenomas and carcinomas. Virchows Arch. 438(6), 595–602 (2001)
C.B. Pizarro, M.C. Oliveira, J.F. Pereira‐Lima, C.G. Leães, C.K. Kramer, T. Schuch, L.M. Barbosa‐Coutinho, N.P. Ferreira, Evaluation of angiogenesis in 77 pituitary adenomas using endoglin as a marker. Neuropathology 29(1), 40–44 (2009)
R.V. Lloyd, S. Vidal, E. Horvath, K. Kovacs, B. Scheithauer, Angiogenesis in normal and neoplastic pituitary tissues. Microscopy Res. Tech. 60(2), 244–250 (2003)
K. Takada, S. Yamada, A. Teramoto, Correlation between tumor vascularity and clinical findings in patients with pituitary adenomas. Endocr. Pathol. 15(2), 131–139 (2004)
M. Raica, M. Coculescu, A.M. Cimpean, D. Ribatti, Endocrine gland derived-VEGF is down-regulated in human pituitary adenoma. Anticancer Res. 30(10), 3981–3986 (2010)
R.V. Lloyd, B.W. Scheithauer, T. Kuroki, S. Vidal, K. Kovacs, L. Stefaneanu, Vascular endothelial growth factor (VEGF) expression in human pituitary adenomas and carcinomas. Endocr. Pathol. 10(3), 229–235 (1999)
J.M. Walz, D. Boehringer, H.L. Deissler, L. Faerber, J.C. Goepfert, P. Heiduschka, S.M. Kleeberger, A. Klettner, T.U. Krohne, N. Schneiderhan-Marra, Pre-analytical parameters affecting vascular endothelial growth factor measurement in plasma: identifying confounders. PLoS One 11(1), e0145375 (2016)
R. Sánchez-Ortiga, L. Sánchez-Tejada, O. Moreno-Perez, P. Riesgo, M. Niveiro, A.M.P. Alfonso, Over-expression of vascular endothelial growth factor in pituitary adenomas is associated with extrasellar growth and recurrence. Pituitary 16(3), 370–377 (2013)
H.E. Turner, J.A. Wass, Are markers of proliferation valuable in the histological assessment of pituitary tumours? Pituitary 1(3-4), 147–151 (1999)
S. Borg, K. Kerry, J. Royds, R. Battersby, T. Jones, Correlation of VEGF production with IL1α and IL6 secretion by human pituitary adenoma cells. Eur. J. Endocrinol. 152(2), 293–300 (2005)
T. Iuchi, N. Saeki, K. Osato, A. Yamaura, Proliferation, vascular endothelial growth factor expression and cavernous sinus invasion in growth hormone secreting pituitary adenomas. Acta Neurochirurgica 142(12), 1345–1351 (2000)
P. Lohrer, J. Gloddek, U. Hopfner, M. Losa, E. Uhl, U. Pagotto, G.K. Stalla, U. Renner, Vascular endothelial growth factor production and regulation in rodent and human pituitary tumor cells in vitro. Neuroendocrinology 74(2), 95–105 (2001)
N. Li, Z. Jiang, Relationship between expression of vascular endothelial growth factor and the proliferation of prolactinomas. Clin. Neurol. Neurosurg. 153, 102–106 (2017)
S. Fukui, H. Nawashiro, N. Otani, H. Ooigawa, A. Yano, N. Nomura, A.M. Tokumaru, T. Miyazawa, A. Ohnuki, N. Tsuzuki, H. Katoh, S. Ishihara, K. Shima, Vascular endothelial growth factor expression in pituitary adenomas. Acta. Neurochir. Suppl. 86, 519–521 (2003)
Y. Kong, Z. Ren, C. Su, R. Wang, B. Xing, Expressive level of vascular endothelial growth factor in peripheral blood in patients with pituitary adenomas. Zhongguo yi xue ke xue yuan xue bao. Acta Academiae Medicinae Sinicae 26(2), 164–167 (2004)
W. He, L. Huang, X. Shen, Y. Yang, D. Wang, Y. Yang, X. Zhu, Relationship between RSUME and HIF-1α/VEGF-A with invasion of pituitary adenoma. Gene 603, 54–60 (2017)
L.-x Pan, Z.-p Chen, Y.-s Liu, J.-h Zhao, Magnetic resonance imaging and biological markers in pituitary adenomas with invasion of the cavernous sinus space. J. Neuro-oncol. 74(1), 71–76 (2005)
A. Sav, F. Rotondo, L.V. Syro, B.W. Scheithauer, K. Kovacs, Biomarkers of pituitary neoplasms. Anticancer Res. 32(11), 4639–4654 (2012)
S. Schreiber, W. Saeger, D.K. Lüdecke, Proliferation markers in different types of clinically non-secreting pituitary adenomas. Pituitary 1(3-4), 213–220 (1999)
V. Sergio, H. Eva, K. Kalman, W. Bernd, R. Scheithauer, V. Llyod, K. George, Ultrastructural features of apoptosis in human pituitary adenomas. Ultrastructural Pathol. 25(2), 85–92 (2001)
F. Matano, D. Yoshida, Y. Ishii, S. Tahara, A. Teramoto, A. Morita, Endocan, a new invasion and angiogenesis marker of pituitary adenomas. J. Neuro-oncol. 117(3), 485–491 (2014)
B.D. Grigoriu, F. Depontieu, A. Scherpereel, D. Gourcerol, P. Devos, T. Ouatas, J.-J. Lafitte, M.-C. Copin, A.-B. Tonnel, P. Lassalle, Endocan expression and relationship with survival in human non–small cell lung cancer. Clin. Cancer Res. 12(15), 4575–4582 (2006)
S. Wang, Z. Wu, L. Wei, J. Zhang, Endothelial cell-specific molecule-1 as an invasiveness marker for pituitary null cell adenoma. BMC Endocr. Disord. 19(1), 90 (2019)
Acknowledgements
We acknowledged the patients participated in this study.
Author contributions
Conceptualization and methodology: M.E.K., M.G., and H.A.; Material preparation: M.G.; Investigation: M.P.-S., and A.Z.M.; Data collection: M.G., H.A., and M.G.; Writing—original draft preparation: M.G.; Writing—review and editing: M.E. K., B.J.-M., and M.H.
Funding
This study was funded by Iran University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The current study was carried out according to Helsinki Declaration and was approved by the ethics committee of Iran University of Medical Sciences (IR.IUMS.FMD.REC1396.9511330003).
Informed consent
Verbal informed consent was obtained from the patients.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary material
Rights and permissions
About this article
Cite this article
Ghadir, M., Khamseh, M.E., Panahi-shamsabad, M. et al. Cell proliferation, apoptosis, and angiogenesis in non-functional pituitary adenoma: association with tumor invasiveness. Endocrine 69, 596–603 (2020). https://doi.org/10.1007/s12020-020-02366-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02366-6