Abstract
Diabetic nephropathy (DN) is a leading cause of end stage renal failure and there is an urgent need to identify new clinical biomarkers and targets for treatment to effectively prevent and slow the progression of the complication. Many lines of evidence show that inflammation is a cardinal pathogenetic mechanism in DN. Studies in animal models of experimental diabetes have demonstrated that there is a low-grade inflammation in the diabetic kidney. Both pharmacological and genetic strategies targeting inflammatory molecules have been shown to be beneficial in experimental DN. In vitro studies have cast light on the cellular mechanisms whereby diabetes triggers inflammation and in turn inflammation magnifies the kidney injury. Translation of this basic science knowledge into potential practical clinical applications is matter of great interest for researchers today. This review focuses on key pro-inflammatory systems implicated in the development of DN: the tumor necrosis factor(TNF)-α/TNF-α receptor system, the monocyte chemoattractant protein-1/CC-chemokine receptor-2 system, and the Endocannabinoid system that have been selected as they appear particularly promising for future clinical applications.


Similar content being viewed by others
References
American Diabetes Association, Standards of medical care in diabetes. Diabetes Care 37(suppl.1), S42–S44 (2014)
L.A. Stevens, T. Greene, A.S. Levey, Surrogate end points for clinical trials of kidney disease progression. Clin. J. Am. Soc. Nephrol. 1, 874–884 (2006)
H.J. Lambers Heerspink, D. de Zeeuw, Debate: PRO position. Should microalbuminuria ever be considered as a renal endpoint in any clinical trial? Am. J. Nephrol. 31, 458–461 (2010)
R.J. Glassock, Debate: CON position. Should microalbuminuria ever be considered as a renal endpoint in any clinical trial? Am. J. Nephrol. 31, 462–465 (2010)
R.J. Macisaac, G. Jerums, Diabetic kidney disease with and without albuminuria. Curr. Opin. Nephrol. Hypertens. 20, 246–257 (2011)
G. Penno, A. Solini, E. Bonora, C. Fondelli, E. Orsi, G. Zerbini, R. Trevisan, M. Vedovato, G. Gruden, F. Cavalot, M. Cignarelli, L. Laviola, S. Morano, A. Nicolucci, G. Pugliese, Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J. Hypertens. 29, 1802–1809 (2011)
DCCT. The effect of intensive treatment of diabetes on the development and progression of long term complications in the insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–86 (1993)
UK Prospective Diabetes Study, UKPDS) Group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33. Lancet 12, 837–853 (1998)
E.J. Lewis, L.G. Hunsicker, R.P. Bain, R.D. Rohde, The effect of ACE inhibition on diabetic nephropathy. N. Engl. J. Med. 329, 1456–1462 (1993)
A.S. Krolewski, M. Canessa, J.H. Warram, L.M. Laffel, A.R. Christlieb, W.C. Knowler, L.I. Rand, Predisposition to hypertension and susceptibility to renal disease in insulin-dependent diabetes mellitus. N. Engl. J. Med. 318, 140 (1988)
P. Gaede, P. Vedel, H.H. Parving, O. Pedersen, Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet 20(353), 617–622 (1999)
T. Furuta, T. Saito, T. Ootaka, J. Soma, K. Obara, K. Abe, K. Yoshinaga, The role of macrophages in diabetic glomerulosclerosis. Am. J. Kidney Dis. 21, 480–485 (1993)
C. Viedt, S.R. Orth, Monocyte chemoattractant protein-1 (MCP-1) in the kidney: does it more than simply attract monocytes? Nephrol. Dial. Transplant. 17, 2043–2047 (2002)
C. Sassy-Prigent, D. Heudes, C. Mandet, M.F. Bélair, O. Michel, B. Perdereau, J. Bariéty, P. Bruneval, Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes 49, 466–475 (2000)
H. Sugimoto, K. Shikata, K. Hirata, K. Akiyama, M. Matsuda, M. Kushiro, Y. Shikata, N. Miyatake, M. Miyasaka, H. Makino, Increased expression of intercellular adhesion molecule-1 (ICAM-1) in diabetic rat glomeruli: glomerular hyperfiltration is a potential mechanism of ICAM-1 upregulation. Diabetes 46, 2075–2081 (1997)
S. Kato, V.A. Luyckx, M. Ots, K.W. Lee, F. Ziai, J.L. Troy, B.M. Brenner, H.S. MacKenzie, Renin-angiotensin blockade lowers MCP-1 expression in diabetic rats. Kidney Int. 56, 1037–1048 (1999)
S. Okada, K. Shikata, M. Matsuda, D. Ogawa, H. Usui, Y. Kido, R. Nagase, J. Wada, Y. Shikata, H. Makino, Intercellular adhesion molecule-1—deficient mice are resistant against renal injury after induction of diabetes. Diabetes 52, 2586–2593 (2003)
F.Y. Chow, D.J. Nikolic-Paterson, E. Ozols, R.C. Atkins, B.J. Rollin, G.H. Tesch, Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 69, 73–80 (2006)
H. You, T. Gao, T.K. Cooper, W. Brian Reeves, A.S. Awad, Macrophages directly mediate diabetic renal injury. Am. J. Physiol. Renal Physiol. 305, F1719–F1727 (2013)
I.Z. Pawluczyk, K.P. Harris, Macrophages promote prosclerotic responses in cultured rat mesangial cells: a mechanism for the initiation of glomerulosclerosis. J. Am. Soc. Nephrol. 8, 1525–1536 (1997)
Y. Ikezumi, T. Suzuki, T. Karasawa, H. Kawachi, D.J. Nikolic-Paterson, M. Uchiyama, Activated macrophages down-regulate podocyte nephrin and podocin expression via stress-activated protein kinases. Biochem. Biophys. Res. Commun. 376, 706–711 (2008)
C.W. Park, J.H. Kim, J.H. Lee, Y.S. Kim, H.J. Ahn, Y.S. Shin, S.Y. Kim, E.J. Choi, Y.S. Chang, B.K. Bang, High glucose-induced intercellular adhesion molecule-1 (ICAM-1) expression through an osmotic effect in rat mesangial cells is PKC-NF-kappa B-dependent. Diabetologia 43, 1544–1553 (2000)
F. Chow, E. Ozols, D.J. Nikolic-Paterson, R.C. Atkins, G.H. Tesch, Macrophage in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 65, 116–128 (2004)
F.Y. Chow, D.J. Nikolic-Paterson, F.Y. Ma, E. Ozols, B.J. Rollins, G.H. Tesch, Monocyte chemoattractant protein 1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia 50, 471–480 (2007)
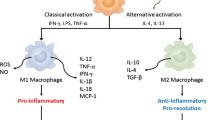
S.D. Ricardo, H. van Goor, A.A. Eddy, Macrophage diversity in renal injury and repair. J. Clin. Invest. 118, 3522–3530 (2008)
D. Zheng, Y. Wang, Q. Cao, V.W. Lee, G. Zheng, Y. Sun, T.K. Tan, Y. Wang, S.I. Alexander, D.C. Harris, Transfused macrophages ameliorate pancreatic and renal injury in murine diabetes mellitus. Nephron Exp. Nephrol. 118, e87–e99 (2011)
P.J. Naudé, J.A. den Boer, P.G. Luiten, U.L. Eisel, Tumor necrosis factor receptor cross-talk. FEBS J. 278, 888–898 (2011)
A. Ortiz, C. Bustos, J. Alonso, R. Alcázar, M.J. López-Armada, J.J. Plaza, E. González, J. Egido, Involvement of tumor necrosis factor-alpha in the pathogenesis of experimental, and human glomerulonephritis. Adv. Nephrol. Necker Hosp. 24, 53–77 (1995)
S.M. Laster, J.G. Wood, L.R. Gooding, Tumor necrosis factor can induce both apoptotic and necrotic forms of cell lysis. J. Immunol. 141, 2629–2634 (1988)
J.J. Boyle, P.L. Weissberg, M.R. Bennett, Tumor necrosis factor-alpha promotes macrophage-induced vascular smooth muscle cell apoptosis by direct and autocrine mechanisms. Arterioscler. Thromb. Vasc. Biol. 23, 1553–1558 (2003)
J.A. McKenzie, A.J. Ridley, Roles of Rho/ROCK and MLCK in TNF-alpha-induced changes in endothelial morphology and permeability. J. Cell Physiol. 213, 221–228 (2007)
H.H. Radeke, B. Meier, N. Topley, J. Flöge, G.G. Habermehl, K. Resch, Interleukin 1-alpha and tumor necrosis factor-alpha induce oxygen radical production mesangial cells. Kidney Int. 37, 767–775 (1990)
G. Hasegawa, K. Nakano, M. Sawada, K. Uno, Y. Shibayama, K. Ienaga, M. Kondo, Possible role of tumor necrosis factor and interleukin-1 in the development of diabetic nephropathy. Kidney Int. 40, 1007–1012 (1991)
L. Baud, J.P. Oudinet, M. Bens, L. Noe, M.N. Peraldi, E. Rondeau, J. Etienne, R. Ardaillou, Production of tumor necrosis factor by rat mesangial cells in response to bacterial lipopolysaccharide. Kidney Int. 35, 1111–1118 (1989)
A.K. Hughes, P.K. Stricklett, D.E. Kohan, Shiga toxin-1 regulation of cytokine production by human glomerular epithelial cells. Nephron. 88, 14–23 (2001)
A.M. Jevnikar, D.C. Brennan, G.G. Singer, J.E. Heng, W. Maslinski, R.P. Wuthrich, L.H. Glimcher, V.E. Kelley, Stimulated kidney tubular epithelial cells express membrane associated and secreted TNF-α. Kidney Int. 40, 203–211 (1991)
X.H. Wu, S.M. Huang, W.X. Fan, W.X. Tang, H.Y. Qiu, Influence of high glucose and mannose binding lectin complement pathway activation to IL-6 and TNF-alpha’s expression by human renal glomerular endothelial cells. Sichuan Da Xue Xue Bao Yi Xue Ban. 42, 90–94. Chinese. (2011)
H. Sugimoto, K. Shikata, J. Wada, S. Horiuchi, H. Makino, Advanced glycation end products-cytokine-nitric oxide sequence pathway in the development of diabetic nephropathy: aminoguanidine ameliorates the overexpression of tumour necrosis factor-alpha and inducible nitric oxide synthase in diabetic rat glomeruli. Diabetologia 42, 878–886 (1999)
K. Omote, T. Gohda, M. Murakoshi, Y. Sasaki, S. Kazuno, T. Fujimura, M. Ishizaka, Y. Sonoda, Y. Tomino, Role of the TNF pathway in the progression of diabetic nephropathy in KK-Ay mice. Am. J. Physiol. Renal Physiol. 306, F1335–F1347 (2014)
K. Kalantarinia, A.S. Awad, H.M. Siragy, Urinary and renal interstitial concentrations of TNF-α increase prior to the rise in albuminuria in diabetic rats. Kidney Int. 64, 1208–1213 (2003)
Y. Moriwaki, T. Inokuchi, A. Yamamoto, T. Ka, Z. Tsutsumi, S. Takahashi, T. Yamamoto, Effect of TNF-α inhibition on urinary albumin excretion in experimental diabetic rats. Acta Diabetol. 44, 215–218 (2007)
R. Pai, B. Bassa, M.A. Kirschenbaum, V.S. Kamanna, TNF-alpha stimulates monocyte adhesion to glomerular mesangial cells. The role of intercellular adhesion molecule-1 gene expression and protein kinases. J. Immunol. 156, 2571–2579 (1996)
S.K. Lee, J.Y. Park, S.J. Chung, W.S. Yang, S.B. Kim, S.K. Park, J.S. Park, Chemokines, osteopontin, ICAM-1 gene expression in cultured rat mesangial cells. J. Korean Med. Sci. 13, 165–170 (1998)
A. Marfaing-Koka, O. Devergne, G. Gorgone, A. Portier, T.J. Schall, P. Galanaud, D. Emilie, Regulation of the production of the RANTES chemokine by endothelial cells. Synergistic induction by IFN-gamma plus TNF-alpha and inhibition by IL-4 and IL-13. J. Immunol. 154, 1870–1878 (1995)
V.S. Kamanna, R. Pai, B. Bassa, M.A. Kirschenbaum, Activation of mesangial cells with TNF-alpha stimulates M-CSF gene expression and monocyte proliferation: evidence for involvement of protein kinase C and protein tyrosine kinase. Biochim. Biophys. Acta 1313, 161–172 (1996)
G. Wolf, S. Aberle, F. Thaiss, P.J. Nelson, A.M. Krensky, E.G. Neilson, R.A. Stahl, TNF alpha induces expression of the chemoattractant cytokine RANTES in cultured mouse mesangial cells. Kidney Int. 44, 795–804 (1993)
C. Zoja, J.M. Wang, S. Bettoni, M. Sironi, D. Renzi, F. Chiaffarino, H.E. Abboud, J. Van Damme, A. Mantovani, G. Remuzzi, Interleukin-1 beta and tumor necrosis factor-alpha induce gene expression and production of leukocyte chemotactic factors, colony-stimulating factors, and interleukin-6 in human mesangial cells. Am. J. Pathol. 138, 991–1003 (1991)
A. Taubitz, M. Schwarz, N. Eltrich, M.T. Lindenmeyer, V. Vielhaue, Distinct contributions of TNF receptor 1 and 2 to TNF-induced glomerular inflammation in mice. PLoS One 8, e68167 (2013)
Z. Hruby, K.F. Beck, Cytotoxic effect of autocrine and macrophage derived nitric oxide on cultured rat mesangial cells. Clin. Exp. Immunol. 107, 76–82 (1997)
I.Z. Pawluczyk, K.P. Harris, Cytokine interactions promote synergistic fibronectin accumulation by mesangial cells. Kidney Int. 54, 62–70 (1998)
N. Koike, T. Takamura, S. Kaneko, Induction of reactive oxygen species from isolated rat glomeruli by protein kinase C activation and TNF- stimulation, and effects of a phosphodiesterase inhibitor. Life Sci. 80, 1721–1728 (2007)
K. Yamauchi, Y. Takano, A. Kasai, K. Hayakawa, N. Hiramatsu, N. Enomoto, J. Yao, M. Kitamura, Screening and identification of substances that regulate nephrin gene expression using engineered reporter podocytes. Kidney Int. 70, 892–900 (2006)
Y. Takano, K. Yamauchi, K. Hayakawa, N. Hiramatsu, A. Kasai, M. Okamura, M. Yokouchi, A. Shitamura, J. Yao, M. Kitamura, Transcriptional suppression of nephrin in podocytes by macrophages: role of inflammatory cytokines and involvement of the PI3K/Akt pathway. FEBS Lett. 581, 421–426 (2007)
S. Doublier, V. Ruotsalainen, G. Salvidio, E. Lupia, L. Biancone, P.G. Conaldi, P. Reponen, K. Tryggvason, G. Camussi, Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am. J. Pathol. 158, 1723–1731 (2001)
S.B. Koukouritaki, E.A. Vardaki, E.A. Papakonstanti, E. Lianos, C. Stournaras, D.S. Emmanouel, TNF-alpha induces actin cytoskeleton reorganization in glomerular epithelial cells involving tyrosine phosphorylation of paxillin and focal adhesion kinase. Mol. Med. 5, 382–392 (1999)
T. Tejada, P. Catanuto, A. Ijaz, J.V. Santos, X. Xia, P. Sanchez, N. Sanabria, O. Lenz, S.J. Elliot, A. Fornoni, Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int. 73, 1385–1393 (2008)
J. Han, P. Thompson, B. Beutler, Dexamethasone and pentoxifylline inhibit endotoxin-induced cachectin/tumor necrosis factor synthesis at separate points in the signalling pathway. J. Exp. Med. 172, 393–394 (1990)
G.M. Doherty, J.C. Jensen, H.R. Alexander, C.M. Buresh, J.A. Norton, Pentoxifylline suppression of tumor necrosis factor gene transcription. Surgery. 110, 192–198 (1991)
M.C. Thomas, Emerging drugs for managing kidney disease in patients with diabetes. Expert Opin. Emerging Drugs. 18, 55–70 (2013)
B.B. McCormick, A. Sydor, A. Akbari, D. Fergusson, S. Doucette, G. Knoll, The effect of pentoxifylline on proteinuria in diabetic kidney disease: a meta-analysis. Am. J. Kidney Dis. 52, 454–463 (2008)
J.F. Navarro-González, C. Mora-Fernández, M. Muros de Fuentes, M.L. Méndez, E. Gallego, M. Macía, N. Del Castillo, A. Rivero, N. Del Castillo, M.A. Getino, P. García, A. Jarque, J. García, Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: the PREDIAN trial. J. Am. Soc. Nephrol. (2014). doi:10.1681/ASN.2014010012
M.H. Bemelmans, L.J. van Tits, W.A. Buurman, Tumor necrosis factor: function, release and clearance. Crit. Rev. Immunol. 16, 1–11 (1996)
Y. Moriwaki, T. Yamamoto, Y. Shibutani, E. Aoki, Z. Tsutsumi, S. Takahashi, H. Okamura, M. Koga, M. Fukuchi, T. Hada, Elevated levels of interleukin-18 and tumor necrosis factor-alpha in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Metabolism. 53, 605–608 (2003)
J.F. Navarro, C. Mora, M. Muros, J. García, Urinary tumour necrosis factor-α excretion independently correlates with clinical markers of glomerular and tubulointerstitial injury in type 2 diabetic patients. Nephrol. Dial. Transplant. 21(12), 3428–3434 (2006)
G. Gruden, F. Barutta, N. Chaturvedi, C. Schalkwijk, C.D. Stehouwer, S. Pinach, M. Manzo, M. Loiacono, M. Tricarico, G. Mengozzi, D.R. Witte, J.H. Fuller, P.C. Perin, G. Bruno, NH2-terminal probrain natriuretic peptide is associated with diabetes complications in the EURODIAB Prospective Complications Study: the role of tumor necrosis factor-α. Diabetes Care 35, 1931–1936 (2012)
J. Lin, F.B. Hu, E.B. Rimm, N. Rifai, G.C. Curhan, The association of serum lipids and inflammatory biomarkers with renal function in men with type II diabetes mellitus. Kidney Int. 69, 336–342 (2006)
M.A. Niewczas, L.H. Ficociello, A.C. Johnson, W. Walker, E.T. Rosolowsky, B. Roshan, J.H. Warram, A.S. Krolewski, Serum concentrations of markers of TNFα and Fas-mediated pathways and renal function in nonproteinuric patients with type 1 diabetes. Clin. J. Am. Soc. Nephrol. 4, 62–70 (2009)
T. Gohda, M.A. Niewczas, L.H. Ficociello, W.H. Walker, J. Skupien, F. Rosetti, X. Cullere, A.C. Johnson, G. Crabtree, A.M. Smiles, T.N. Mayadas, J.H. Warram, A.S. Krolewski, Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J. Am. Soc. Nephrol. 23, 516–524 (2012)
M.A. Niewczas, T. Gohda, J. Skupien, A.M. Smiles, W.H. Walker, F. Rosetti, X. Cullere, J.H. Eckfeldt, A. Doria, T.N. Mayadas, J.H. Warram, A.S. Krolewski, Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J. Am. Soc. Nephrol. 23, 507–515 (2012)
M.F. Lopes-Virella, N.L. Baker, K.J. Hunt, P.A. Cleary, R. Klein, G. Virella, DCCT/EDIC Research Group.: Baseline markers of inflammation are associated with progression to macroalbuminuria in type 1 diabetic subjects. Diabetes Care 36, 2317–2323 (2013)
C. Forsblom, J. Moran, V. Harjutsalo, T. Loughman, J. Wadén, N. Tolonen, L. Thorn, M. Saraheimo, D. Gordin, P.H. Groop, M.C. Thomas, on behalf of the FinnDiane Study Group, Added value of soluble Tumor Necrosis Factor Alpha Receptor-1 as a biomarker of ESRD risk. Diabetes Care (2014). doi:10.2337/dc14-0225
J. Lin, F.B. Hu, C. Mantzoros, G.C. Curhan, Lipid and inflammatory biomarkers and kidney function decline in type 2 diabetes. Diabetologia 53, 263–267 (2010)
F.T. Lee, Z. Cao, D.M. Long, S. Panagiotopoulos, G. Jerums, M.E. Cooper, J.M. Forbes, Interactions between angiotensin II and NF-kappaB-dependent pathways in modulating macrophage infiltration in experimental diabetic nephropathy. J. Am. Soc. Nephrol. 15, 2139–2151 (2004)
S. Mezzano, A. Droguett, M.E. Burgos, L.G. Ardiles, C.A. Flores, C.A. Aros, I. Caorsi, C.P. Vío, M. Ruiz-Ortega, J. Egido, Renin-angiotensin system activation and interstitial inflammation in human diabetic nephropathy. Kidney Int. Suppl. 86, S64–S70 (2003)
E. Tarabra, S. Giunti, F. Barutta, G. Salvidio, D. Burt, G. Deferrari, R. Gambino, D. Vergola, S. Pinach, P.C. Perin, G. Camussi, G. Gruden, Effect of the monocyte chemoattractant protein-1/CC chemokine receptor 2 system on nephrin expression in streptozotocin-treated mice and human cultured podocytes. Diabetes 58, 2109–2118 (2009)
N. Banba, T. Nakamura, M. Matsumura, H. Kuroda, Y. Hattori, K. Kasai, Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. Kidney Int. 58, 684–690 (2000)
H. Ha, M.R. Yu, Y.J. Choi, M. Kitamura, H.B. Lee, Role of high glucose–induced nuclear factor-B activation in monocyte chemoattractant protein-1 expression by mesangial cells. J. Am. Soc. Nephrol. 13, 894–902 (2002)
M. Naito, A. Shenoy, I. Aoyama, J.S. Koopmeiners, R. Komers, H.W. Schnaper, K. Bomsztyk, High ambient glucose augments angiotensin ii-induced proinflammatory gene mrNA expression in human mesangial cells: effects of valsartan and simvastatin. Am. J. Nephrol. 30, 99–111 (2009)
C.G. Ihm, J.K. Park, S.P. Hong, T.W. Lee, B.S. Cho, M.J. Kim, D.R. Cha, H. Ha, A high glucose concentration stimulates the expression of monocyte chemotactic peptide 1 in human mesangial cells. Nephron. 79, 33–37 (1998)
S.Y. Han, G.A. So, Y.H. Jee, K.H. Han, Y.S. Kang, H.K. Kim, S.W. Kang, D.S. Han, J.Y. Han, D.R. Cha, Effect of retinoic acid in experimental diabetic nephropathy. Immunol. Cell Biol. 82, 568–576 (2004)
E.Y. Lee, C.H. Chung, C.C. Khoury, T.K. Yeo, P.E. Pyagay, A. Wang, S. Chen, The monocyte chemoattractant protein-1/CCR2 loop, inducible by TGF-beta, increases podocyte motility and albumin permeability. Am. J. Physiol. Renal Physiol. 297, F85–F94 (2009)
T. Matsui, S. Yamagishi, M. Takeuchi, S. Ueda, K. Fukami, S. Okuda, Nifedipine, a calcium channel blocker, inhibits advanced glycation end product (AGE)-elicited mesangial cell damage by suppressing AGE receptor (RAGE) expression via peroxisome proliferatoractivated receptor-gamma activation. Biochem. Biophys. Res. Commun. 385, 269–272 (2009)
T. Matsui, S. Yamagishi, S. Ueda, K. Nakamura, T. Imaizumi, M. Takeuchi, H. Inoue, Telmisartan, an angiotensin II type 1 receptor blocker, inhibits advanced glycation end-product (AGE)-induced monocyte chemoattractant protein-1 expression in mesangial cells through downregulation of receptor for AGEs via peroxisome proliferator-activated receptor-gamma activation. J. Int. Med. Res. 35, 482–489 (2007)
L. Gu, S. Hagiwara, Q. Fan, M. Tanimoto, M. Kobata, M. Yamashita, T. Nishitani, T. Gohda, Z. Ni, J. Qian, S. Horikoshi, Y. Tomino, Role of receptor for advanced glycation end-products and signalling events in advanced glycation end-product-induced monocyte chemoattractant protein-1 expression in differentiated mouse podocytes. Nephrol. Dial. Transplant. 21, 299–313 (2006)
G. Gruden, G. Setti, A. Hayward, D. Sugden, S. Duggan, D. Burt, R.E. Buckingham, L. Gnudi, G. Viberti, Mechanical stretch induces monocyte chemoattractant activity via an NF-kappaB-dependent monocyte chemoattractant protein-1-mediated pathway in human mesangial cells: inhibition by rosiglitazone. J. Am. Soc. Nephrol. 16, 688–696 (2005)
J. Cheng, M.M. Diaz Encarnacion, G.M. Warner, C.E. Gray, K.A. Nath, J.P. Grande, TGF-beta1 stimulates monocyte chemoattractant protein-1 expression in mesangial cells through a phosphodiesterase isoenzyme 4-dependent process. Am. J. Physiol. Cell Physiol. 289, C959–C970 (2005)
W. Qi, X. Chen, T.S. Polhill, S. Sumual, S. Twigg, R.E. Gilbert, C.A. Pollock, TGF-β1 induces IL-8 and MCP-1 through a connective tissue growth factor-independent pathway. Am. J. Physiol. Renal Physiol. 290, F703–F709 (2006)
B.H. Rovin, T. Yoshiumura, L. Tan, Cytokine-induced production of monocyte chemoattractant protein-1 by cultured human mesangial cells. J. Immunol. 148, 2148–2153 (1992)
Y. Watanabe, M. Tamura, A. Osajima, H. Anai, N. Kabashima, R. Serino, Y. Nakashima, Integrins induce expression of monocyte chemoattractant protein-1 via focal adhesion kinase in mesangial cells. Kidney Int. 64, 431–440 (2003)
A.A. Eddy, C.M. Giachelli, Renal expression of genes that promote interstitial inflammation and fibrosis in rats with protein-overload proteinuria. Kidney Int. 47, 1546–1557 (1995)
Y. Wang, J. Chen, L. Chen, Y.C. Tay, G.K. Rangan, D.C. Harris, Induction of monocyte chemoattractant protein-1 in proximal tubule cells by urinary protein. J. Am. Soc. Nephrol. 8, 1537–1545 (1997)
S. Giunti, Targeting the MCP-1/CCR2 system in diabetic kidney disease. Curr. Vasc. Pharmacol. 8, 849–860 (2010)
S. Giunti, F. Barutta, P.C. Perin, G. Gruden, The MCP-1/CCR2 system has direct proinflammatory effects in human mesangial cells. Kidney Int. 69, 856–863 (2006)
D. Burt, G. Salvidio, E. Tarabra, F. Barutta, S. Pinach, P. Dentelli, G. Camussi, P.C. Perin, G. Gruden, The monocyte chemoattractant protein-1/cognate CC chemokine receptor 2 system affects cell motility in cultured human podocytes. Am. J. Pathol. 171, 1789–1799 (2007)
V.H. Rao, D.T. Meehan, D. Delimont, M. Nakajima, T. Wada, M.A. Gratton, D. Cosgrove, Role for macrophage metalloelastase in glomerular basement membrane damage associated with alport syndrome. Am. J. Pathol. 169, 32–46 (2006)
S. Giunti, G.H. Tesch, S. Pinach, D.J. Burt, M.E. Cooper, P. Cavallo-Perin, G. Camussi, G. Gruden, Monocyte chemoattractant protein-1 has prosclerotic effects both in a mouse model of experimental diabetes and in vitro in human mesangial cells. Diabetologia 51, 198–207 (2008)
J. Park, D.R. Ryu, J.J. Li, D.S. Jung, S.J. Kwak, S.H. Lee, T.H. Yoo, S.H. Han, J.E. Lee, D.K. Kim, S.J. Moon, K. Kim, D.S. Han, S.W. Kang, MCP-1/CCR2 system is involved in high glucose-induced fibronectin and type IV collagen expression in cultured mesangial cells. Am. J. Physiol. Renal Physiol. 295, F749–F757 (2008)
B.Y. Nam, J. Paeng, S.H. Kim, S.H. Lee, H. do Kim, H.Y. Kang, J.J. Li, S.J. Kwak, J.T. Park, T.H. Yoo, S.H. Han, D.K. Kim, S.W. Kang, The MCP-1/CCR2 axis in podocytes is involved in apoptosis induced by diabetic conditions. Apoptosis 17, 1–13 (2012)
H. Kanamori, T. Matsubara, A. Mima, E. Sumi, K. Nagai, T. Takahashi, H. Abe, N. Iehara, A. Fukatsu, H. Okamoto, T. Kita, T. Doi, H. Arai, Inhibition of MCP-1/CCR2 pathway ameliorates the development of diabetic nephropathy. Biochem. Biophys. Res. Commun. 360, 772–777 (2007)
P. Celec, J. Hodosy, R. Gardlík, M. Behuliak, R. Pálffy, M. Pribula, P. Jáni, J. Turňa, K. Sebeková, The effects of anti-Inflammatory and anti-angiogenic DNA vaccination on diabetic nephropathy in rats. Hum. Gene Ther. 23, 158–166 (2012)
A.S. Awad, G.R. Kinsey, K. Khutsishvili, T. Gao, W.K. Bolton, M.D. Okusa, Monocyte/macrophage chemokine receptor CCR2 mediates diabetic renal injury. Am. J. Physiol. Renal Physiol. 301, F1358–F1366 (2011)
S.J. Seok, E.S. Lee, G.T. Kim, M. Hyun, J.H. Lee, S. Chen, R. Choi, H.M. Kim, E.Y. Lee, C.H. Chung, Blockade of CCL2/CCR2 signalling ameliorates diabetic nephropathy in db/db mice. Nephrol. Dial. Transplant. 28, 1700–1710 (2013)
M. Okamoto, M. Fuchigami, T. Suzuki, N. Watanabe, A novel C-C chemokine receptor 2 antagonist prevents progression of albuminuria and atherosclerosis in mouse models. Biol. Pharm. Bull. 35, 2069–2074 (2012)
S.G. Sayyed, M. Ryu, O.P. Kulkarni, H. Schmid, J. Lichtnekert, S. Grüner, L. Green, P. Mattei, G. Hartmann, H.J. Anders, An orally active chemokine receptor CCR2 antagonist prevents glomerulosclerosis and renal failure in type 2 diabetes. Kidney Int. 80, 68–78 (2011)
T.J. Sullivan, Z. Miao, D.J. Dairaghi, A. Krasinski, Y. Wang, B.N. Zhao, T. Baumgart, L.S. Ertl, A. Pennell, L. Seitz, J. Powers, R. Zhao, S. Ungashe, Z. Wei, L. Boring, C.L. Tsou, I. Charo, R.D. Berahovich, T.J. Schall, J.C. Jaen, CCR2 antagonist CCX140-B provides renal and glycemic benefits in diabetic transgenic human CCR2 knockin mice. Am. J. Physiol. Renal Physiol. 305, F1288–F1297 (2013)
T.J. Sullivan, Z. Miao, B.N. Zhao, L.S. Ertl, Y. Wang, A. Krasinski, M.J. Walters, J.P. Powers, D.J. Dairaghi, T. Baumgart, L.C. Seitz, R.D. Berahovich, T.J. Schall, J.C. Jaen, Experimental evidence for the use of CCR2 antagonists in the treatment of type 2 diabetes. Metab. Clin. Exp. 62, 1623–1632 (2013)
J.F. Navarro-González, C. Mora-Fernández, M. de Muros Fuentes, J. García-Pérez, Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 7, 327–340 (2011)
M. Hanefeld, E. Schell, I. Gouni-Berthold, M. Melichar, I. Vesela, D. Johnson, S. Miao, T.J. Sullivan, J.C. Jaen, T.J. Schall, P. Bekker, the CCX140-B Diabetes Study Group, Orally-administered chemokine receptor CCR2 antagonist CCX140-B in type 2 diabetes: a pilot double-blind, randomized clinical trial. J. Diabetes Metabolism. 3, 9 (2012)
V. Ninichuk, S. Clauss, O. Kulkarni, H. Schmid, S. Segerer, E. Radomska, D. Eulberg, K. Buchner, N. Selve, S. Klussmann, H.J. Anders, Late onset of Ccl2 blockade with the Spiegelmer mNOX-E36-3’PEG prevents glomerulosclerosis and improves glomerular filtration rate in db/db mice. Am. J. Pathol. 172, 628–637 (2008)
S. Kiyici, E. Erturk, F. Budak, C. Ersoy, E. Tuncel, C. Duran, B. Oral, D. Sigirci, S. Imamoglu, Serum monocyte chemoattractant protein-1 and monocyte adhesion molecules in type 1 diabetic patients with nephropathy. Arch. Med. Res. 37, 998–1003 (2006)
T. Wada, K. Furuichi, N. Sakai, Y. Iwata, K. Yoshimoto, M. Shimizu, S.I. Takeda, K. Takasawa, M. Yoshimura, H. Kida, K.I. Kobayashi, N. Mukaida, T. Naito, K. Matsushima, H. Yokoyama, Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int. 58, 1492–1499 (2000)
N.G. Frangogiannis, The prognostic value of monocyte chemoattractant protein-1/CCL2 in acute coronary syndromes. J. Am. Coll. Cardiol. 50, 2125–2127 (2007)
T. Morii, H. Fujita, T. Narita, T. Shimotomai, H. Fujishima, N. Yoshioka, H. Imai, M. Kakei, S. Ito, Association of monocyte chemoattractant protein-1 with renal tubular damage in diabetic nephropathy. J. Diabetes Complicat. 17(1), 11–15 (2003)
F.W. Tam, B.L. Riser, K. Meeran, J. Rambow, C.D. Pusey, A.H. Frankel, Urinary monocyte chemoattractant protein-1 (MCP-1) and connective tissue growth factor (CCN2) as prognostic markers for progression of diabetic nephropathy. Cytokine 47, 37–42 (2009)
M. Kano, T. Ohno-Shosaku, Y. Hashimotodani, M. Uchigashima, M. Watanabe, Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 9, 309–380 (2009)
J. Tam, J. Liu, B. Mukhopadhyay, R. Cinar, G. Godlewski, G. Kunos, Endocannabinoids in liver disease. Hepatology 53, 346–355 (2011)
P. Pacher, P. Mukhopadhyay, R. Mohanraj, G. Godlewski, S. Bátkai, G. Kunos, Modulation of the endocannabinoid system in cardiovascular disease: therapeutic potential and limitations. Hypertension 52, 601–607 (2008)
C. Silvestri, V. Di Marzo, The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 17, 475–490 (2013)
N. Leleu-Chavain, M. Body-Malapel, J. Spencer, P. Chavatte, P. Desreumaux, R. Millet, Recent advances in the development of selective CB(2) agonists as promising anti-inflammatory agents. Curr. Med. Chem. 19, 3457–3474 (2012)
F. Barutta, F. Piscitelli, S. Pinach, G. Bruno, R. Gambino, M.P. Rastaldi, G. Salvidio, V. Di Marzo, P. Cavallo Perin, G. Gruden, Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy. Diabetes 60, 2386–2396 (2011)
F. Barutta, A. Corbelli, R. Mastrocola, R. Gambino, V. Di Marzo, S. Pinach, M.P. Rastaldi, P.C. Perin, G. Gruden, Cannabinoid receptor 1 blockade ameliorates albuminuria in experimental diabetic nephropathy. Diabetes 59, 1046–1054 (2010)
K.H. Han, S. Lim, J. Ryu, C.W. Lee, Y. Kim, J.H. Kang, S.S. Kang, Y.K. Ahn, C.S. Park, J.J. Kim, CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc. Res. 84, 378–386 (2009)
D.H. Nam, Blockade of cannabinoid receptor 1 improves insulin resistance, lipid metabolism, and diabetic nephropathy in db/db mice. Endocrinology 153, 1387–1396 (2012)
J.C. Lim, M.H. Lee, J.E. Kim, H.K. Song, Y.S. Kang, J.E. Lee, H.W. Kim, J.J. Cha, Y.Y. Hyun, S.H. Kim, S.Y. Han, K.H. Han, J.Y. Han, D.R. Cha, Cannabinoid receptor 1 mediates high glucose-induced apoptosis via endoplasmic reticulum stress in primary cultured rat mesangial cells. Am. J. Physiol. Renal Physiol. 301, F179–F188 (2011)
F. Barutta, S. Grimaldi, I. Franco, S. Bellini, R. Gambino, S. Pinach, A. Corbelli, G. Bruno, M.P. Rastaldi, T. Aveta, E. Hirsch, V. Di Marzo, G. Gruden, Deficiency of cannabinoid receptor of type 2 worsens renal functional and structural abnormalities in streptozotocin-induced diabetic mice. Kidney Int. (2014). doi:10.1038/ki.2014.165
K.A. Jenkin, A.J. McAinch, J.F. Briffa, Y. Zhang, D.J. Kelly, C.A. Pollock, P. Poronnik, D.H. Hryciw, Cannabinoid receptor 2 expression in human proximal tubule cells is regulated by albumin independent of ERK1/2 signaling. Cell. Physiol. Biochem. 32, 1309–1319 (2013)
P. Janiak, B. Poirier, J.P. Bidouard, C. Cadrouvele, F. Pierre, L. Gouraud, I. Barbosa, J. Dedio, J.P. Maffrand, G. Le Fur, S. O’Connor, J.M. Herbert, Blockade of cannabinoid CB1 receptor improves renal function, metabolic profile, and increased survival of obese Zucker rats. Kidney Int. 72, 1345–1357 (2007)
F. Montecucco, F. Burger, F. Mach, S. Steffens, CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am. J. Physiol. Heart Circ. Physiol. 294, H1145–H1155 (2008)
J. Tam, V.K. Vemuri, J. Liu, S. Bátkai, B. Mukhopadhyay, G. Godlewski, D. Osei-Hyiaman, S. Ohnuma, S.V. Ambudkar, J. Pickel, A. Makriyannis, G. Kunos, Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J. Clin. Invest. 120, 2953–2966 (2010)
S. Steffens, N.R. Veillard, C. Arnaud, G. Pelli, F. Burger, C. Staub, M. Karsak, A. Zimmer, J.L. Frossard, F. Mach, Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 434, 782–786 (2005)
G.D. Duerr, J.C. Heinemann, G. Suchan, E. Kolobara, D. Wenzel, C. Geisen, M. Matthey, K. Passe-Tietjen, W. Mahmud, A. Ghanem, K. Tiemann, J. Alferink, S. Burgdorf, R. Buchalla, A. Zimmer, B. Lutz, A. Welz, B.K. Fleischmann, O. Dewald, The endocannabinoid-CB2 receptor axis protects the ischemic heart at the early stage of cardiomyopathy. Basic Res. Cardiol. 109, 425 (2014)
E.J. Rahn, A.G. Hohmann, Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics. 6, 713–737 (2009)
P. Pacher, R. Mechoulam, Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog. Lipid Res. 50, 193–211 (2011)
V. Deveaux, T. Cadoudal, Y. Ichigotani, F. Teixeira-Clerc, A. Louver, S. Manin, J. Tran-Van Nhieu, M.P. Belot, A. Zimmer, P. Even, P.D. Cani, C. Knauf, R. Burcelin, A. Bertola, Y. Le Marchand-Brustel, P. Gual, A. Mallat, S. Lotersztajn, Cannabinoid CB2 receptor potentiates obesity-associated inflammation, Insulin resistance and hepatic steatosis. PloS One. 4(6), e5844 (2009)
Acknowledgments
This work was supported by the European Federation for the Study of Diabetes, the Compagnia di San Paolo, and the University of Turin.
Conflict of interest
The authors declare that they do not have conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barutta, F., Bruno, G., Grimaldi, S. et al. Inflammation in diabetic nephropathy: moving toward clinical biomarkers and targets for treatment. Endocrine 48, 730–742 (2015). https://doi.org/10.1007/s12020-014-0437-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-014-0437-1