Abstract
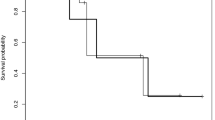
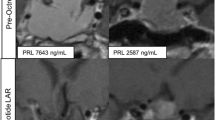
As ErbB signaling is a determinant of prolactin synthesis, role of ErbB receptors was tested for prolactinoma outcomes and therapy. The objective of this study was to characterize ErbB receptor expression in prolactinomas and then perform a pilot study treating resistant prolactinomas with a targeted tyrosine kinase inhibitor (TKI). Retrospective analysis of prolactinomas and pilot study for dopamine agonist resistant prolactinomas in tertiary referral center. We performed immunofluorescent staining of a tissue array of 29 resected prolactinoma tissues for EGFR, ErbB2, ErbB3, and ErbB4 correlated with clinical features. Two patients with aggressive resistant prolactinomas enrolled and completed trial. They received lapatinib 1,250 mg daily for 6 months with tumor and hormone assessments. Main outcome measures were positive tumor staining of respective ErbB receptors, therapeutic reduction of prolactin levels and tumor shrinkage. Treated PRL levels and tumor volumes were suppressed in both subjects treated with TKI. EGFR expression was positive in 82 % of adenomas, ErbB2 in 92 %, ErbB3 in 25 %, and ErbB4 in 71 %, with ErbB2 score > EGFR > ErbB4 > ErbB3. Higher ErbB3 expression was associated with optic chiasm compression (p = 0.03), suprasellar extension (p = 0.04), and carotid artery encasement (p = 0.01). Higher DA response rates were observed in tumors with higher ErbB3 expression. Prolactinoma expression of specific ErbB receptors is associated with tumor invasion, symptoms, and response to dopamine agonists. Targeting ErbB receptors may be effective therapy in patients with resistant prolactinomas.




Similar content being viewed by others
References
S. Melmed, Mechanisms for pituitary tumorigenesis: the plastic pituitary. J. Clin. Invest. 112(11), 1603–1618 (2003)
A. Di Sarno, M.L. Landi, P. Cappabianca, F. Di Salle, F.W. Rossi, R. Pivonello, C. Di Somma, A. Faggiano, G. Lombardi, A. Colao, Resistance to cabergoline as compared with bromocriptine in hyperprolactinemia: prevalence, clinical definition, and therapeutic strategy. J. Clin. Endocrinol. Metab. 86(11), 5256–5261 (2001)
T. Mancini, F.F. Casanueva, A. Giustina, Hyperprolactinemia and prolactinomas. Endocrinol. Metab. Clin. N. Am. 37(1), 67–99 (2008). doi:10.1016/j.ecl.2007.10.013
S. Melmed, F.F. Casanueva, A.R. Hoffman, D.L. Kleinberg, V.M. Montori, J.A. Schlechte, J.A. Wass, Diagnosis and treatment of hyperprolactinemia: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 96(2), 273–288 (2011). doi:10.1210/jc.2010-1692
F.F. Casanueva, M.E. Molitch, J.A. Schlechte, R. Abs, V. Bonert, M.D. Bronstein, T. Brue, P. Cappabianca, A. Colao, R. Fahlbusch, H. Fideleff, M. Hadani, P. Kelly, D. Kleinberg, E. Laws, J. Marek, M. Scanlon, L.G. Sobrinho, J.A. Wass, A. Giustina, Guidelines of the pituitary society for the diagnosis and management of prolactinomas. Clin. Endocrinol. 65(2), 265–273 (2006). doi:10.1111/j.1365-2265.2006.02562.x
M. Losa, P. Mortini, R. Barzaghi, L. Gioia, M. Giovanelli, Surgical treatment of prolactin-secreting pituitary adenomas: early results and long-term outcome. J. Clin. Endocrinol. Metab. 87(7), 3180–3186 (2002)
O. Serri, E. Rasio, H. Beauregard, J. Hardy, M. Somma, Recurrence of hyperprolactinemia after selective transsphenoidal adenomectomy in women with prolactinoma. N. Engl. J. Med. 309(5), 280–283 (1983). doi:10.1056/NEJM198308043090505
G. Zada, W.W. Woodmansee, S. Ramkissoon, J. Amadio, V. Nose, E.R. Laws Jr, Atypical pituitary adenomas: incidence, clinical characteristics, and implications. J. Neurosurg. 114(2), 336–344 (2011). doi:10.3171/2010.8.JNS10290
G. Raverot, A. Wierinckx, E. Dantony, C. Auger, G. Chapas, L. Villeneuve, T. Brue, D. Figarella-Branger, P. Roy, E. Jouanneau, M. Jan, J. Lachuer, J. Trouillas, Prognostic factors in prolactin pituitary tumors: clinical, histological, and molecular data from a series of 94 patients with a long postoperative follow-up. J. Clin. Endocrinol. Metab. 95(4), 1708–1716 (2010). doi:10.1210/jc.2009-1191
S. Melmed, Pathogenesis of pituitary tumors. Nat. Rev. Endocrinol. 7(5), 257–266 (2011). doi:10.1038/nrendo.2011.40
G.A. Kaltsas, P. Nomikos, G. Kontogeorgos, M. Buchfelder, A.B. Grossman, Clinical review: diagnosis and management of pituitary carcinomas. J. Clin. Endocrinol. Metab. 90(5), 3089–3099 (2005)
B.W. Scheithauer, O. Kurtkaya-Yapicier, K.T. Kovacs, W.F. Young Jr, R.V. Lloyd, Pituitary carcinoma: a clinicopathological review. Neurosurgery 56(5), 1066–1074 (2005); discussion 1066–1074
M.P. Gillam, M.E. Molitch, G. Lombardi, A. Colao, Advances in the treatment of prolactinomas. Endocr. Rev. 27(5), 485–534 (2006)
M.D. Bronstein, M. Knoepfelmacher, B. Liberman, R. Marino Jr, O.A. Germek, A.V. Schally, Absence of suppressive effect of somatostatin on prolactin levels in patients with hyperprolactinemia. Horm. Metab. Res. 19(6), 271–274 (1987). doi:10.1055/s-2007-1011796
S.W. Lamberts, T. Verleun, R. Oosterom, Effect of tamoxifen administration on prolactin release by invasive prolactin-secreting pituitary adenomas. Neuroendocrinology 34(5), 339–342 (1982)
G. Raverot, N. Sturm, F. de Fraipont, M. Muller, S. Salenave, P. Caron, O. Chabre, P. Chanson, C. Cortet-Rudelli, R. Assaker, H. Dufour, S. Gaillard, P. Francois, E. Jouanneau, J.G. Passagia, M. Bernier, A. Cornelius, D. Figarella-Branger, J. Trouillas, F. Borson-Chazot, T. Brue, Temozolomide treatment in aggressive pituitary tumors and pituitary carcinomas: a French multicenter experience. J. Clin. Endocrinol. Metab. 95(10), 4592–4599 (2010). doi:10.1210/jc.2010-0644
M. Losa, E. Mazza, M.R. Terreni, A. McCormack, A.J. Gill, M. Motta, M.G. Cangi, A. Talarico, P. Mortini, M. Reni, Salvage therapy with temozolomide in patients with aggressive or metastatic pituitary adenomas: experience in six cases. Eur. J. Endocrinol. 163(6), 843–851 (2010). doi:10.1530/EJE-10-0629
N.E. Hynes, H.A. Lane, ERBB receptors and cancer: the complexity of targeted inhibitors. Nat. Rev. Cancer 5(5), 341–354 (2005)
Y. Yarden, M.X. Sliwkowski, Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2(2), 127–137 (2001)
R. Roskoski Jr, The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem. Biophys. Res. Commun. 319(1), 1–11 (2004)
V. Gorgoulis, D. Aninos, P. Mikou, P. Kanavaros, A. Karameris, J. Joardanoglou, A. Rasidakis, M. Veslemes, B. Ozanne, D.A. Spandidos, Expression of EGF, TGF-alpha and EGFR in squamous cell lung carcinomas. Anticancer Res. 12(4), 1183–1187 (1992)
J.S. Ross, J.A. Fletcher, The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells 16(6), 413–428 (1998). doi:10.1002/stem.160413
W. Xia, Y.K. Lau, H.Z. Zhang, F.Y. Xiao, D.A. Johnston, A.R. Liu, L. Li, R.L. Katz, M.C. Hung, Combination of EGFR, HER-2/neu, and HER-3 is a stronger predictor for the outcome of oral squamous cell carcinoma than any individual family members. Clin. Cancer Res. 5(12), 4164–4174 (1999)
R.J. Gilbertson, R.H. Perry, P.J. Kelly, A.D. Pearson, J. Lunec, Prognostic significance of HER2 and HER4 coexpression in childhood medulloblastoma. Cancer Res. 57(15), 3272–3280 (1997)
H. Zhang, A. Berezov, Q. Wang, G. Zhang, J. Drebin, R. Murali, M.I. Greene, ErbB receptors: from oncogenes to targeted cancer therapies. J. Clin. Invest. 117(8), 2051–2058 (2007)
D.A. Cameron, S. Stein, Drug Insight: intracellular inhibitors of HER2: clinical development of lapatinib in breast cancer. Nat. Clin. Pract. Oncol. 5(9), 512–520 (2008). doi:10.1038/ncponc1156
G.H. Murdoch, E. Potter, A.K. Nicolaisen, R.M. Evans, M.G. Rosenfeld, Epidermal growth factor rapidly stimulates prolactin gene transcription. Nature 300(5888), 192–194 (1982)
A. Mouihate, J. Lestage, Epidermal growth factor: a potential paracrine and autocrine system within the pituitary. NeuroReport 6(10), 1401–1404 (1995)
J. Armstrong, G.V. Childs, Changes in expression of epidermal growth factor receptors by anterior pituitary cells during the estrous cycle: cyclic expression by gonadotropes. Endocrinology 138(5), 1903–1908 (1997)
K. Zhang, E. Kulig, L. Jin, R.V. Lloyd, Effects of estrogen and epidermal growth factor on prolactin and Pit-1 mRNA in GH3 cells. Proc. Soc. Exp. Biol. Med. 202(2), 193–200 (1993)
C.A. Pickett, N. Manning, Y. Akita, A. Gutierrez-Hartmann, Role of specific protein kinase C isozymes in mediating epidermal growth factor, thyrotropin-releasing hormone, and phorbol ester regulation of the rat prolactin promoter in GH4/GH4C1 pituitary cells. Mol. Endocrinol. 16(12), 2840–2852 (2002)
J. Hapgood, T.A. Libermann, I. Lax, Y. Yarden, A.B. Schreiber, Z. Naor, J. Schlessinger, Monoclonal antibodies against epidermal growth factor receptor induce prolactin synthesis in cultured rat pituitary cells (GH3). Proc. Natl Acad. Sci. USA 80(21), 6451–6455 (1983)
H. Fukuoka, O. Cooper, J. Mizutani, Y. Tong, S.G. Ren, S. Bannykh, S. Melmed, HER2/ErbB2 receptor signaling in rat and human prolactinoma cells: strategy for targeted prolactinoma therapy. Mol. Endocrinol. 25(1), 92–103 (2011). doi:10.1210/me.2010-0353
G. Vlotides, E. Siegel, I. Donangelo, S. Gutman, S.G. Ren, S. Melmed, Rat prolactinoma cell growth regulation by epidermal growth factor receptor ligands. Cancer Res. 68(15), 6377–6386 (2008). doi:10.1158/0008-5472.CAN-08-0508
G. Vlotides, O. Cooper, Y.H. Chen, S.G. Ren, Y. Greenman, S. Melmed, Heregulin regulates prolactinoma gene expression. Cancer Res. 69(10), 4209–4216 (2009). doi:10.1158/0008-5472.CAN-08-4934
R. Leake, D. Barnes, S. Pinder, I. Ellis, L. Anderson, T. Anderson, R. Adamson, T. Rhodes, K. Miller, R. Walker, Immunohistochemical detection of steroid receptors in breast cancer: a working protocol. UK Receptor Group, UK NEQAS, The Scottish Breast Cancer Pathology Group, and The Receptor and Biomarker Study Group of the EORTC. J. Clin. Pathol. 53(8), 634–635 (2000)
E. Charafe-Jauffret, C. Tarpin, V.J. Bardou, F. Bertucci, C. Ginestier, A.C. Braud, B. Puig, J. Geneix, J. Hassoun, D. Birnbaum, J. Jacquemier, P. Viens, Immunophenotypic analysis of inflammatory breast cancers: identification of an ‘inflammatory signature’. J. Pathol. 202(3), 265–273 (2004). doi:10.1002/path.1515
E. Knosp, E. Steiner, K. Kitz, C. Matula, Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33(4), 610–617 (1993); discussion 617–618
M. Al-Shraim, S.L. Asa, The 2004 World Health Organization classification of pituitary tumors: what is new? Acta Neuropathol. 111(1), 1–7 (2006). doi:10.1007/s00401-005-1093-6
F.L. Chen, W. Xia, N.L. Spector, Acquired resistance to small molecule ErbB2 tyrosine kinase inhibitors. Clin. Cancer Res. 14(21), 6730–6734 (2008). doi:10.1158/1078-0432.CCR-08-0581
M. Sanchez-Martin, A. Pandiella, Differential action of small molecule HER kinase inhibitors on receptor heterodimerization: therapeutic implications. Int. J. Cancer 131(1), 244–252 (2012). doi:10.1002/ijc.26358
M. Scaltriti, C. Verma, M. Guzman, J. Jimenez, J.L. Parra, K. Pedersen, D.J. Smith, S. Landolfi, S. Ramon y Cajal, J. Arribas, J. Baselga, Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene 28(6), 803–814 (2009). doi:10.1038/onc.2008.432
J.T. Garrett, M.G. Olivares, C. Rinehart, N.D. Granja-Ingram, V. Sanchez, A. Chakrabarty, B. Dave, R.S. Cook, W. Pao, E. McKinely, H.C. Manning, J. Chang, C.L. Arteaga, Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc. Natl Acad. Sci. USA 108(12), 5021–5026 (2011). doi:10.1073/pnas.1016140108
G. Kontogeorgos, L. Stefaneanu, K. Kovacs, Z. Cheng, Localization of epidermal growth factor (EGF) and epidermal growth factor receptor (EGFr) in human pituitary adenomas and nontumorous pituitaries: an immunocytochemical study. Endocr. Pathol. 7(1), 63–70 (1996)
M.L. Jaffrain-Rea, E. Petrangeli, C. Lubrano, G. Minniti, D. Di Stefano, F. Sciarra, L. Frati, G. Tamburrano, G. Cantore, A. Gulino, Epidermal growth factor binding sites in human pituitary macroadenomas. J. Endocrinol. 158(3), 425–433 (1998)
M. Theodoropoulou, T. Arzberger, Y. Gruebler, M.L. Jaffrain-Rea, J. Schlegel, L. Schaaf, E. Petrangeli, M. Losa, G.K. Stalla, U. Pagotto, Expression of epidermal growth factor receptor in neoplastic pituitary cells: evidence for a role in corticotropinoma cells. J. Endocrinol. 183(2), 385–394 (2004)
O. Onguru, B.W. Scheithauer, K. Kovacs, S. Vidal, L. Jin, S. Zhang, K.H. Ruebel, R.V. Lloyd, Analysis of epidermal growth factor receptor and activated epidermal growth factor receptor expression in pituitary adenomas and carcinomas. Mod. Pathol. 17(7), 772–780 (2004)
S.S. Chaidarun, M.C. Eggo, M.C. Sheppard, P.M. Stewart, Expression of epidermal growth factor (EGF), its receptor, and related oncoprotein (erbB-2) in human pituitary tumors and response to EGF in vitro. Endocrinology 135(5), 2012–2021 (1994)
S. Ezzat, L. Zheng, H.S. Smyth, S.L. Asa, The c-erbB-2/neu proto-oncogene in human pituitary tumours. Clin. Endocrinol. (Oxf) 46(5), 599–606 (1997)
V. Nose-Alberti, M.I. Mesquita, L.C. Martin, M.J. Kayath, Adrenocorticotropin-producing pituitary carcinoma with expression of c-erbB-2 and high PCNA index: a comparative study with pituitary adenomas and normal pituitary tissues. Endocr. Pathol. 9(1), 53–62 (1998)
C.H. Botelho, A.V. Magalhaes, P.A. Mello, F.C. Schmitt, L.A. Casulari, Expression of p53, Ki-67 and c-erb B2 in growth hormone-and/or prolactin-secreting pituitary adenomas. Arq. Neuropsiquiatr. 64(1), 60–66 (2006)
H. Fukuoka, O. Cooper, A. Ben-Shlomo, A. Mamelak, S.G. Ren, D. Bruyette, S. Melmed, EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J. Clin. Invest. 121(12), 4712–4721 (2011). doi:10.1172/JCI60417
C. Missale, L. Castelletti, F. Boroni, M. Memo, P. Spano, Epidermal growth factor induces the functional expression of dopamine receptors in the GH3 cell line. Endocrinology 128(1), 13–20 (1991)
J. Kim, H. Jeong, Y. Lee, C. Kim, H. Kim, A. Kim, HRG-beta1-driven ErbB3 signaling induces epithelial-mesenchymal transition in breast cancer cells. BMC Cancer 13, 383 (2013). doi:10.1186/1471-2407-13-383
G.D. Plowman, J.M. Culouscou, G.S. Whitney, J.M. Green, G.W. Carlton, L. Foy, M.G. Neubauer, M. Shoyab, Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc. Natl Acad. Sci. USA 90(5), 1746–1750 (1993)
Acknowledgments
This work was supported by NIH Grant K23DK085148 (O. Cooper). Dr. Shlomo Melmed for continuing mentorship, guidance, and patient consultations. GlaxoSmithKline for provision of lapatinib, Kolja Wawrowsky for confocal imaging of TMA and advise, Grace Labrado and Karen Geiser for assistance with preparation of the manuscript, Lori Korsakoff and Billy Gellepis for coordinating the research study.
Disclosures
The authors have no disclosures.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The studies in this manuscript were approved and monitored by the Institutional Review Board.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
12020_2013_93_MOESM3_ESM.eps
Supplemental Fig. 1: Representative samples of intensity scores 0–3 in prolactinoma tissues (stained for ErbB2) ×20 magnification. a Intensity score 0; b intensity score 1; c intensity score 2 and d intensity score 3 (EPS 48058 kb)
Rights and permissions
About this article
Cite this article
Cooper, O., Mamelak, A., Bannykh, S. et al. Prolactinoma ErbB receptor expression and targeted therapy for aggressive tumors. Endocrine 46, 318–327 (2014). https://doi.org/10.1007/s12020-013-0093-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-013-0093-x