Abstract
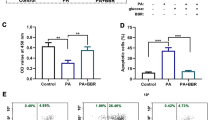
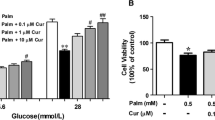
The purpose of this study is to explore the possible link between oxidative stress and endoplasmic reticulum (ER) stress in palmitate (PA) induced apoptosis of INS-1 cells, and to figure out the main source of reactive oxygen species (ROS) and the effect of ROS inhibition on the level of ER stress. In this study, INS-1 cells were exposed to PA and oleate for the indicated times. Cell viability and apoptosis were measured by MTT and ELISA; ROS was detected by the probe DCFH-DA and MitoSOX Red using flow cytometer; and the ER stress-related chaperones were measured by western blotting and real time PCR. The level of JNK phosphorylation was also measured by western blotting. The results showed that, in PA-treated cells, apoptosis increased in a dose-dependent way. ROS generation was mainly increased through mitochondrion, and ROS inhibition reduced the expression of some ER chaperones and transcription factors levels. Also, inhibition of JNK phosphorylation ameliorated PA-induced apoptosis. It is concluded that, ROS inhibition, especially inhibiting the ROS from mitochondria, may reduce the expression of some ER stress-related effectors and show a protective role in PA-induced pancreatic beta-cell apoptosis.






Similar content being viewed by others
References
S.A. Westphal, Obesity, abdominal obesity, and insulin resistance. Clin. Cornerstone. 9(1), 23–29 (2008). discussion 30–21
S.E. Kahn, The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 46(1), 3–19 (2003). doi:10.1007/s00125-002-1009-0
I. Kim, W. Xu, J.C. Reed, Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 7(12), 1013–1030 (2008). doi:10.1038/nrd2755
J. Chandra, A. Samali, S. Orrenius, Triggering and modulation of apoptosis by oxidative stress. Free Radic. Biol. Med. 29(3–4), 323–333 (2000)
F. Giacco, M. Brownlee, Oxidative stress and diabetic complications. Circ. Res. 107(9), 1058–1070 (2010). doi:10.1161/CIRCRESAHA.110.223545
F. Zheng, W. Lu, C. Jia, H. Li, Z. Wang, W. Jia, Relationships between glucose excursion and the activation of oxidative stress in patients with newly diagnosed type 2 diabetes or impaired glucose regulation. Endocrine 37(1), 201–208 (2010). doi:10.1007/s12020-009-9296-6
C. Fleury, B. Mignotte, J.L. Vayssiere, Mitochondrial reactive oxygen species in cell death signaling. Biochimie 84(2–3), 131–141 (2002)
J.D. Lambeth, NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4(3), 181–189 (2004). doi:10.1038/nri1312
M.D. Brand, C. Affourtit, T.C. Esteves, K. Green, A.J. Lambert, S. Miwa, J.L. Pakay, N. Parker, Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic. Biol. Med. 37(6), 755–767 (2004). doi:10.1016/j.freeradbiomed.2004.05.034
S. Lenzen, J. Drinkgern, M. Tiedge, Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 20(3), 463–466 (1996)
S. Nakamura, T. Takamura, N. Matsuzawa-Nagata, H. Takayama, H. Misu, H. Noda, S. Nabemoto, S. Kurita, T. Ota, H. Ando, K. Miyamoto, S. Kaneko, Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J. Biol. Chem. 284(22), 14809–14818 (2009). doi:10.1074/jbc.M901488200
J. Ishiyama, R. Taguchi, Y. Akasaka, S. Shibata, M. Ito, M. Nagasawa, K. Murakami, Unsaturated FAs prevent palmitate-induced LOX-1 induction via inhibition of ER stress in macrophages. J. Lipid Res. 52(2), 299–307 (2011). doi:10.1194/jlr.M007104
S.G. Fonseca, J. Gromada, F. Urano, Endoplasmic reticulum stress and pancreatic beta-cell death. Trends. Endocrinol. Metab. 22(7), 266–274 (2011). doi:10.1016/j.tem.2011.02.008
D.L. Eizirik, A.K. Cardozo, M. Cnop, The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 29(1), 42–61 (2008). doi:10.1210/er.2007-0015
J.A. Martinez, Mitochondrial oxidative stress and inflammation: an slalom to obesity and insulin resistance. J. Physiol. Biochem. 62(4), 303–306 (2006)
M. Cnop, L. Ladriere, M. Igoillo-Esteve, R.F. Moura, D.A. Cunha, Causes and cures for endoplasmic reticulum stress in lipotoxic beta-cell dysfunction. Diabetes Obes. Metab. 12(Suppl 2), 76–82 (2010). doi:10.1111/j.1463-1326.2010.01279.x
G.X. Shen, Oxidative stress and diabetic cardiovascular disorders: roles of mitochondria and NADPH oxidase. Can. J. Physiol. Pharmacol. 88(3), 241–248 (2010). doi:10.1139/Y10-018
H. Kaneto, T.A. Matsuoka, Y. Nakatani, D. Kawamori, T. Miyatsuka, M. Matsuhisa, Y. Yamasaki, Oxidative stress, ER stress, and the JNK pathway in type 2 diabetes. J. Mol. Med. (Berl). 83(6), 429–439 (2005). doi:10.1007/s00109-005-0640-x
H. Kaneto, Y. Nakatani, D. Kawamori, T. Miyatsuka, T.A. Matsuoka, M. Matsuhisa, Y. Yamasaki, Role of oxidative stress, endoplasmic reticulum stress, and c-Jun N-terminal kinase in pancreatic beta-cell dysfunction and insulin resistance. Int. J. Biochem. Cell Biol. 38(5–6), 782–793 (2006)
E. Karaskov, C. Scott, L. Zhang, T. Teodoro, M. Ravazzola, A. Volchuk, Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology 147(7), 3398–3407 (2006). doi:10.1210/en.2005-1494
I. Kharroubi, L. Ladriere, A.K. Cardozo, Z. Dogusan, M. Cnop, D.L. Eizirik, Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology 145(11), 5087–5096 (2004). doi:10.1210/en.2004-0478
E. Bachar, Y. Ariav, M. Ketzinel-Gilad, E. Cerasi, N. Kaiser, G. Leibowitz, Glucose amplifies fatty acid-induced endoplasmic reticulum stress in pancreatic beta-cells via activation of mTORC1. PLoS One 4(3), e4954 (2009). doi:10.1371/journal.pone.0004954
G.C. Yaney, B.E. Corkey, Fatty acid metabolism and insulin secretion in pancreatic beta cells. Diabetologia 46(10), 1297–1312 (2003). doi:10.1007/s00125-003-1207-4
M. Cnop, J.C. Hannaert, A. Hoorens, D.L. Eizirik, D.G. Pipeleers, Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes 50(8), 1771–1777 (2001)
A.I. Oprescu, G. Bikopoulos, A. Naassan, E.M. Allister, C. Tang, E. Park, H. Uchino, G.F. Lewis, I.G. Fantus, M. Rozakis-Adcock, M.B. Wheeler, A. Giacca, Free fatty acid-induced reduction in glucose-stimulated insulin secretion: evidence for a role of oxidative stress in vitro and in vivo. Diabetes 56(12), 2927–2937 (2007). doi:10.2337/db07-0075
G. Li, C. Scull, L. Ozcan, I. Tabas, NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J. Cell Biol. 191(6), 1113–1125 (2010). doi:10.1083/jcb.201006121
M.M. Desouki, M. Kulawiec, S. Bansal, G.M. Das, K.K. Singh, Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biol. Ther. 4(12), 1367–1373 (2005)
S.B. Lee, I.H. Bae, Y.S. Bae, H.D. Um, Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. J. Biol. Chem. 281(47), 36228–36235 (2006). doi:10.1074/jbc.M606702200
K. Komiya, T. Uchida, T. Ueno, M. Koike, H. Abe, T. Hirose, R. Kawamori, Y. Uchiyama, E. Kominami, Y. Fujitani, H. Watada, Free fatty acids stimulate autophagy in pancreatic beta-cells via JNK pathway. Biochem. Biophys. Res. Commun. 401(4), 561–567 (2010). doi:10.1016/j.bbrc.2010.09.101
C. Xu, B. Bailly-Maitre, J.C. Reed, Endoplasmic reticulum stress: cell life and death decisions. J. Clin. Invest. 115(10), 2656–2664 (2005). doi:10.1172/JCI26373
S. Chakravarthi, C.E. Jessop, N.J. Bulleid, The role of glutathione in disulphide bond formation and endoplasmic-reticulum-generated oxidative stress. EMBO Rep 7(3), 271–275 (2006). doi:10.1038/sj.embor.7400645
L. Weiss, S. Bernstein, R. Jones, R. Amunugama, D. Krizman, L. Jebailey, O. Almogi-Hazan, Z. Yekhtin, R. Shiner, I. Reibstein, E. Triche, S. Slavin, R. Or, E.R. Barnea, Preimplantation factor (PIF) analog prevents type I diabetes mellitus (TIDM) development by preserving pancreatic function in NOD mice. Endocrine 40(1), 41–54 (2011). doi:10.1007/s12020-011-9438-5
E. Lai, T. Teodoro, A. Volchuk, Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology (Bethesda) 22, 193–201 (2007). doi:10.1152/physiol.00050.2006
F. Urano, X. Wang, A. Bertolotti, Y. Zhang, P. Chung, H.P. Harding, D. Ron, Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287(5453), 664–666 (2000)
H. Kaneto, G. Xu, N. Fujii, S. Kim, S. Bonner-Weir, G.C. Weir, Involvement of c-Jun N-terminal kinase in oxidative stress-mediated suppression of insulin gene expression. J. Biol. Chem. 277(33), 30010–30018 (2002). doi:10.1074/jbc.M202066200
S.C. Martinez, K. Tanabe, C. Cras-Meneur, N.A. Abumrad, E. Bernal-Mizrachi, M.A. Permutt, Inhibition of Foxo1 protects pancreatic islet beta-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes 57(4), 846–859 (2008). doi:10.2337/db07-0595
R. Lupi, F. Dotta, L. Marselli, S. Del Guerra, M. Masini, C. Santangelo, G. Patane, U. Boggi, S. Piro, M. Anello, E. Bergamini, F. Mosca, U. Di Mario, S. Del Prato, P. Marchetti, Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes 51(5), 1437–1442 (2002)
K. Eitel, H. Staiger, J. Rieger, H. Mischak, H. Brandhorst, M.D. Brendel, R.G. Bretzel, H.U. Haring, M. Kellerer, Protein kinase C delta activation and translocation to the nucleus are required for fatty acid-induced apoptosis of insulin-secreting cells. Diabetes 52(4), 991–997 (2003)
C.E. Wrede, L.M. Dickson, M.K. Lingohr, I. Briaud, C.J. Rhodes, Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic beta-cells (INS-1). J. Biol. Chem. 277(51), 49676–49684 (2002). doi:10.1074/jbc.M208756200
J.D. Johnson, Z. Han, K. Otani, H. Ye, Y. Zhang, H. Wu, Y. Horikawa, S. Misler, G.I. Bell, K.S. Polonsky, RyR2 and calpain-10 delineate a novel apoptosis pathway in pancreatic islets. J. Biol. Chem. 279(23), 24794–24802 (2004). doi:10.1074/jbc.M401216200
M. Yamauchi, K. Tsuruma, S. Imai, T. Nakanishi, N. Umigai, M. Shimazawa, H. Hara, Crocetin prevents retinal degeneration induced by oxidative and endoplasmic reticulum stresses via inhibition of caspase activity. Eur. J. Pharmacol. 650(1), 110–119 (2011). doi:10.1016/j.ejphar.2010.09.081
Acknowledgment
This study was supported by the National Natural Science Foundation of China (No. 30872727 and No. 81170757).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, N., Chen, H., Zhang, H. et al. Mitochondrial reactive oxygen species (ROS) inhibition ameliorates palmitate-induced INS-1 beta cell death. Endocrine 42, 107–117 (2012). https://doi.org/10.1007/s12020-012-9633-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-012-9633-z