Abstract
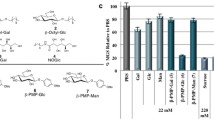
Antifreeze proteins (AFPs) have important functions in many freeze-tolerant organisms. The proteins non-colligatively lower the freezing point and functionally inhibit ice recrystallization in frozen solutions. In our previous studies, we found that the Arctic yeast Leucosporidium sp. produces an AFP (LeIBP), and that the protein could be successfully produced in Pichia expression system. The present study showed that recombinant LeIBP possesses the ability to reduce the damage induced to red blood cells (RBCs) by freeze thawing. In addition to 40 % glycerol, both 0.4 and 0.8 mg/ml LeIBPs significantly reduced freeze–thaw-induced hemolysis at either rapid- (45 °C) or slow-warming (22 °C) temperatures. Post-thaw cell counts of the cryopreserved RBCs were dramatically enhanced, in particular, in 0.8 mg/ml LeIBP. Interestingly, the cryopreserved cells in the presence of LeIBP showed preserved cell size distribution. These results indicate that the ability of LeIBP to inhibit ice recrystallization helps the RBCs avoid critically damaging electrolyte concentrations, which are known as solution effects. Considering all these data, LeIBP can be thought of as a key component in improving RBC cryopreservation efficiency.





Similar content being viewed by others
References
Davies, P. L., Baardsnes, J., Kuiper, M. J., & Walker, V. K. (2002). Structure and function of antifreeze proteins. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 357, 927–935.
Jia, Z., & Davies, P. L. (2002). Antifreeze proteins: an unusual receptor–ligand interaction. Trends in Biochemical Sciences, 27, 101–106.
Knight, C. A., & Duman, J. G. (1986). Inhibition of recrystallization of ice by insect thermal hysteresis proteins: a possible cryoprotective role. Cryobiology, 23, 256–262.
Knight, C. A., DeVries, A. L., & Oolman, L. D. (1984). Fish antifreeze protein and the freezing and recrystallization of ice. Nature, 308, 295–296.
Duman, J., & Horwath, K. (1983). The role of hemolymph proteins in the cold tolerance of insects. Annual Review of Physiology, 45, 261–270.
Urrutia, M. E., Duman, J. G., & Knight, C. A. (1992). Plant thermal hysteresis proteins. Biochimica et Biophysica Acta, 1121, 199–206.
DeVries, A. L. (1983). Antifreeze peptides and glycopeptides in cold-water fishes. Annual Review of Physiology, 45, 245–260.
Davies, P. L., & Hew, C. L. (1990). Biochemistry of fish antifreeze proteins. FASEB Journal, 4, 2460–2468.
DeVries, A. L., & Cheng, C. H. C. (1992). The role of antifreeze glycopropeptides and peptides in the survival of cold-water fishes. Berlin: Springer.
Yu, S. O., Brown, A., Middleton, A. J., Tomczak, M. M., Walker, V. K., & Davies, P. L. (2010). Ice restructuring inhibition activities in antifreeze proteins with distinct differences in thermal hysteresis. Cryobiology, 61, 327–334.
Kristiansen, E., & Zachariassen, K. E. (2005). The mechanism by which fish antifreeze proteins cause thermal hysteresis. Cryobiology, 51, 262–280.
DeVries, A. L., & Wohlschlag, D. E. (1969). Freezing resistance in some Antarctic fishes. Science, 163, 1073–1075.
Chen, G., & Jia, Z. (1999). Ice-binding surface of fish type III antifreeze. Biophysical Journal, 77, 1602–1608.
Low, W. K., Lin, Q., Stathakis, C., Miao, M., Fletcher, G. L., & Hew, C. L. (2001). Isolation and characterization of skin-type, type I antifreeze polypeptides from the longhorn sculpin, Myoxocephalus octodecemspinosus. Journal of Biological Chemistry, 276, 11582–11589.
Deng, G., & Laursen, R. A. (1998). Isolation and characterization of an antifreeze protein from the longhorn sculpin, Myoxocephalus octodecimspinosis. Biochimica et Biophysica Acta, 1388, 305–314.
Hew, C. L., Fletcher, G. L., & Ananthanarayanan, V. S. (1980). Antifreeze proteins from the shorthorn sculpin, Myoxocephalus scorpius: isolation and characterization. Canadian Journal of Biochemistry, 58, 377–383.
Hew, C. L., Wang, N. C., Yan, S., Cai, H., Sclater, A., & Fletcher, G. L. (1986). Biosynthesis of antifreeze polypeptides in the winter flounder. Characterization and seasonal occurrence of precursor polypeptides. European Journal of Biochemistry, 160, 267–272.
Yamashita, Y., Miura, R., Takemoto, Y., Tsuda, S., Kawahara, H., & Obata, H. (2003). Type II antifreeze protein from a mid-latitude freshwater fish, Japanese smelt (Hypomesus nipponensis). Bioscience, Biotechnology, and Biochemistry, 67, 461–466.
Atici, O., & Nalbantoglu, B. (2003). Antifreeze proteins in higher plants. Phytochemistry, 64, 1187–1196.
Kawahara, H., Iwanaka, Y., Higa, S., Muryoi, N., Sato, M., Honda, M., et al. (2007). A novel, intracellular antifreeze protein in an antarctic bacterium, Flavobacterium xanthum. Cryo Letters, 28, 39–49.
Raymond, J. A., Fritsen, C., & Shen, K. (2007). An ice-binding protein from an Antarctic sea ice bacterium. FEMS Microbiology Ecology, 61, 214–221.
Gilbert, J. A., Davies, P. L., & Laybourn-Parry, J. (2005). A hyperactive, Ca2+-dependent antifreeze protein in an Antarctic bacterium. FEMS Microbiology Letters, 245, 67–72.
Xiao, N., Suzuki, K., Nishimiya, Y., Kondo, H., Miura, A., Tsuda, S., et al. (2010). Comparison of functional properties of two fungal antifreeze proteins from Antarctomyces psychrotrophicus and Typhula ishikariensis. FEBS Journal, 277, 394–403.
Duman, J. G. (2001). Antifreeze and ice nucleator proteins in terrestrial arthropods. Annual Review of Physiology, 63, 327–357.
Graether, S. P., & Sykes, B. D. (2004). Cold survival in freeze-intolerant insects: the structure and function of beta-helical antifreeze proteins. European Journal of Biochemistry, 271, 3285–3296.
Lee, J. K., Park, K. S., Park, S., Park, H., Song, Y. H., Kang, S. H., et al. (2010). An extracellular ice-binding glycoprotein from an Arctic psychrophilic yeast. Cryobiology, 60, 222–228.
Rous, P., & Turner, J. R. (1916). The preservation of living red blood cells in vitro: I. Methods of preservation. Journal of Experimental Medicine, 23, 219–237.
Hess, J. R. (2006). An update on solutions for red cell storage. Vox Sanguinis, 91, 13–19.
Hess, J. R., & Greenwalt, T. G. (2002). Storage of red blood cells: new approaches. Transfusion Medicine Reviews, 16, 283–295.
Hill, H. R., Oliver, C. K., Lippert, L. E., Greenwalt, T. J., & Hess, J. R. (2001). The effects of polyvinyl chloride and polyolefin blood bags on red blood cells stored in a new additive solution. Vox Sanguinis, 81, 161–166.
Heaton, W. A., Holme, S., Smith, K., Brecher, M. E., Pineda, A., AuBuchon, J. P., et al. (1994). Effects of 3-5 log10 pre-storage leucocyte depletion on red cell storage and metabolism. British Journal of Haematology, 87, 363–368.
Dumont, L. J., & AuBuchon, J. P. (2008). Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion, 48, 1053–1060.
Pegg, D. E. (1987). Mechanisms of freezing damage. Cambridge: The Company of Biologists Ltd.
Pegg, D. E. (2007). Cryopreservation and freeze-drying protocols (2nd ed.). Totowa: Humana Press.
Smith, A. U. (1950). Prevention of haemolysis during freezing and thawing of red blood-cells. Lancet, 2, 910–911.
Meryman, H. T., & Hornblower, M. (1972). A method for freezing and washing red blood cells using a high glycerol concentration. Transfusion, 12, 145–156.
Rowe, A. W., Eyster, E., & Kellner, A. (1968). Liquid nitrogen preservation of red blood cells for transfusion; a low glycerol-rapid freeze procedure. Cryobiology, 5, 119–128.
Chao, H., Davies, P. L., & Carpenter, J. F. (1996). Effects of antifreeze proteins on red blood cell survival during cryopreservation. Journal of Experimental Biology, 199, 2071–2076.
Carpenter, J. F., & Hansen, T. N. (1992). Antifreeze protein modulates cell survival during cryopreservation: mediation through influence on ice crystal growth. Proceedings of the National Academy of Sciences of the United States of America, 89, 8953–8957.
Kang, J. S., & Raymond, J. A. (2004). Reduction of freeze-thaw-induced hemolysis of red blood cells by an algal ice-binding protein. Cryo Letters, 25, 307–310.
Park, K. S., Lee, J. H., Park, S. I., Do, H., Kim, E. J., Kang, S. H. & Kim, H. J. (2012) Characterization of a homodimeric ice-binding protein from Leucosporidium sp. Cryobiology, 64, 286–296.
Drabkin, D. L., & Austin, J. H. (1935). Spectrophotometric studies. II. Preparations from washed blood cells; nitric oxide hemoglobin and sulfhemoglobin. Journal of Biological Chemistry, 112, 51–65.
Runck, A. H., Valeri, C. R., & Sampson, W. T. (1968). Comparison of the effects of ionic and non-ionic solutions on the volume and intracellular potassium of frozen and non-frozen human red cells. Transfusion, 8, 9–18.
Smallwood, M., Worrall, D., Byass, L., Elias, L., Ashford, D., Doucet, C. J., et al. (1999). Isolation and characterization of a novel antifreeze protein from carrot (Daucus carota). Biochemical Journal, 340(Pt 2), 385–391.
Schmid, P., Huvard, M. J., Lee-Stroka, A. H., Lee, J. Y., Byrne, K. M., & Flegel, W. A. (2011). Red blood cell preservation by droplet freezing with polyvinylpyrrolidone or sucrose-dextrose and by bulk freezing with glycerol. Transfusion, 51(12), 2703–2708.
Hess, J. R. (2010). Red cell storage. Journal of Proteomics, 73, 368–373.
Zimrin, A. B., & Hess, J. R. (2009). Current issues relating to the transfusion of stored red blood cells. Vox Sanguinis, 96, 93–103.
Lovelock, J. E. (1953). The mechanism of the protective action of glycerol against haemolysis by freezing and thawing. Biochimica et Biophysica Acta, 11, 28–36.
Acknowledgments
The authors wish to thank So Ra Yoon for helping in blood collection. This work was supported by grants from Korea Polar Research Institute (PE11100) and Korea Research Council of Fundamental Science and Technology (PG11010 and PG12010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Sung Gu Lee and Hye Yeon Koh contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Lee, S.G., Koh, H.Y., Lee, J.H. et al. Cryopreservative Effects of the Recombinant Ice-Binding Protein from the Arctic Yeast Leucosporidium sp. on Red Blood Cells. Appl Biochem Biotechnol 167, 824–834 (2012). https://doi.org/10.1007/s12010-012-9739-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9739-z