Abstract
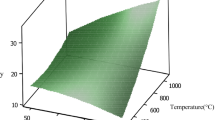
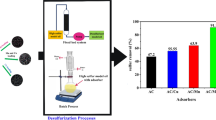
Commercial diesel is often rich with organosulfur compounds and a value of 7,100 mgS/kg was recently reported. As confirmed by chromatographic analysis, about 36% of sulfur compounds are originated from dibenzothiophene. Following uncommon desulfurization method, organosulfur compounds were efficiently removed upon diesel acidification by organic acids prior to activated carbon adsorption. Protonation of S-containing compounds has enhanced their uptake by activated carbon. Competitive adsorption of di/tri/tetra-aromatics and dibenzothiophene from synthetic fuel proved that the later solute was preferentially removed against other aromatics upon fuel acidification. Results showed that 48% of organosulfur compounds were eliminated upon adding acetic acid to a final content of 3% by vol.. Principal component analysis indicated that acid content and carbon mass are the most significant factors on organosulfur compounds removal: %Removal=5.8 (Acid Content)+6.3 (Mass)-0.02 (PD)-0.90 (Temp). The practical efficiency of the proposed method was demonstrated by removing organosulfur compounds from commercial diesel.
Similar content being viewed by others
References
S. Z. Abghari, S. Shokri, B. Baloochi, M. A. Marvast, S. Ghanizadeh and A. Behroozi, Korean J. Chem. Eng., 28, 93 (2011).
M. Seredych, Y. Lison, U. Jans and T. J. Bandosz, Carbon, 47, 2491 (2009).
S. Lee, R. Kumar and M. Krumpelt, Sep. Purif. Technol., 26, 247 (2002).
H. Kim, J. J. Lee and S. H. Moon, Appl. Catal. B: Environ., 44, 287 (2003).
A. Srivastav and V.C. Srivastava, J. Hazard. Mater., 170, 1133 (2009).
V. M. Bhandari, C. H. Ko, J. G. Park, S. Han, S. Cho and J. Kim, Chem. Eng. Sci., 61, 2599 (2006).
A. J. Hernandez-Maldonado and R.T. Yang, Ind. Eng. Chem. Res., 43, 1081 (2004).
F. Mustafa, M.A. Al-Ghouti, F. I. Khalili and Y. S. Al-Degs, J. Hazard. Mater., 182, 97 (2010).
K. S. Kim, J. O Park, J. H. Lee, T. H. Jun and I. Kim, Environ. Eng. Res., 18, 229 (2013).
M. Muzic, K. Sertic-Bionda, Z. Gomzi, S. Podolski and S. Telen, Chem. Eng. Res. Design, 88, 487 (2010).
Y.A. Alhamed and H. S. Bamufleh, Fuel, 88, 87 (2009).
J. Bu, G. Loh, C. Gwie, S. Dewiyanti, M. Tasrif and A. Borgna, Chem. Eng. J., 166, 207 (2011).
C.O. Ania and T. J. Bandosz, Carbon, 44, 2404 (2006).
ASTM D2622–08 Standard Test Method for Sulfur in Petroleum Products by Wavelength Dispersive X-ray Fluorescence Spectrometry. Volume 15.01.
Jordanian Institution for Standard and Metrology (JS 195/2004). Petroleum and Petroleum Products–Automotive fuels–Diesel oil (2004).
M. Jiang, T.N. Flora, R. Ataur and P. Viral, Thermo. Chem. Acta, 434, 27 (2005).
Y. Yang, H. Lu, P. Ying, Z. Jiang and C. Li, Carbon, 45, 3042 (2007).
A. El-Sheikh, Y. Al-Degs, R.M. Al-As’ad and J.A. Sweileh, Desalination, 270, 214 (2011).
A. A. Issa, Y. S. Al-Degs, M.A. Al-Ghouti and A.M. Olimat, Chem. Eng. J., 240, 554 (2014).
M. I. El-Barghouthi, A. H. El-Sheikh, Yahya S. Al-Degs and G. M. Walker, Sep. Sci. Technol., 42, 2195 (2007).
Y. S. Al-Degs, A. H. El-Sheikh, A. A. Issa, M.A. Al-Ghouti and M. Sunjuk, Water Sci. Technol., 66, 1647 (2012).
C. Yu, J. Qiu, Y. Sun, X. Li, G. Chen and Z. Zhao, J. Porous Mater., 15, 151 (2008).
R. G. Brereton, Chemometrics: Data Analysis for the Laboratory and Chemical Plant, Wiley (2003).
R. Dhoble, S. Lunge, A. Bhole and S. Rayalu, Water Res., 45, 4769 (2011).
C. Namasivayam and D. Kavitha, Dyes Pigm., 54, 47 (2002).
S. Faust and O. Aly, Adsorption processes for water treatment, Butterworth Publishers, USA (1987).
M. A. Fazal, A. S. Haseeb and H.H. Masjuki, Energy Conserv. Manage., 67, 251 (2012).
UNE EN-590, Automotive fuels-diesel-requirements and test methods (2004).
ASTM D 975-06, Standard specification for diesel fuel oils.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Ghouti, M.A., Al-Degs, Y.S. A novel desulfurization practice based on diesel acidification prior to activated carbon adsorption. Korean J. Chem. Eng. 32, 685–693 (2015). https://doi.org/10.1007/s11814-014-0303-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-014-0303-0